Abstract
Large number of Chinese people have taken PET/CT health screening during the last decade, resulting in thousands of PET/CT images of healthy subjects stored in the hospital databases all over the country. The purpose of this study is to collect PET/CT images of healthy Chinese people and construct digital atlases of trunk region based on a relatively large sample set. Compared to the traditional digital human atlases built from cryosection image of a single subject, the atlases of this study include anatomical and functional information of various living subjects. The technique of statistical shape models is used to model the inter-subject organ shape deformation across the population, therefore the atlas is named “the deformable atlas”. This study also aims to measure the anatomical parameters (from CT images) and functional metabolism parameters (from PET images) of Chinese adults with different sexes, ages, and weights. The reconstructed statistical shape models reveal major anatomical variations among the population. We also found significant differences between male and female in different age groups through statistical analysis of organs volumes and CT values in skeleton. The obtained models and parameters will support the applications of education, anatomy-based simulation, knowledge-based medical image analysis and etc.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the last decade, digital human atlases of human anatomy have been developed rapidly with the advancement of medical imaging technologies such as CT, MRI, PET and SPECT. Various types of atlases have been constructed focusing on different aspects of human-centered studies. Some atlases aim at providing whole-body scale anatomy, such as the visible human project [1], 3D adult phantoms [2,3,4], pregnant-female models [5] and hybrid new born phantoms [6]. Some atlases focus more on specific organs like liver [7], heart [8], pelvis [9], the femur [10], the vertebrae [11]. In recent years, the Human Brain Project motivated the research on brain atlases based on different imaging modalities [12].
The atlases proposed in this paper belongs to the trunk region category. The trunk atlases can be used as anatomical references to be registered with different modalities of target images or as digital phantoms for radiation simulation. However, most of the existing whole-body scale human atlas were constructed based on single or several references subject in a static pose. It is hard to use such atlases to represent a subject with realistic anatomical variations. To compensate the limitation of subject number, the Virtual Population models including ten detailed high-resolution anatomical models created from whole-body MR images [13]. The atlases mentioned above belong to visible human atlas family which models human anatomy in three dimension. Because visible human atlas is a collection of geometric models of human organ structures, no functional or physiological information is incorporated into the atlas.
With the development of digital human modeling, virtual physiological human has been proposed successively. The goal of virtual physiological human is to digitalize physiological information and integrate it into the visible human in order to reflect growth and metabolism of human body. For organ and system level, the European Physiome Project established a functional model of human digestive and musculoskeletal system [14]. However, none of these atlases can incorporate both organ metabolism information and inter-subject anatomical geometry variations into one single model. Overall, the problem of whole-body scale anatomical variance has not been solved completely, leaving a gap in automated medical image analysis to be filled.
To tackle the above problem, a deformable Chinese human trunk atlas is proposed in this paper. This atlas is constructed based on a training set of PET/CT images of healthy Chinese adults. Compared to the existing human atlas, this atlas adapts its organ anatomy using statistical shape models (SSMs) method. Besides, we obtained the metabolism information in major organs through standard uptake values (SUVs) [15] of radioactive tracer from PET images. With this study, we obtained the statistics of anatomical and functional parameters of the sampled population, as well as the inter-subject anatomical variations of major trunk organs. This trunk atlas is also a preliminary result of a whole-body scale atlas which is to be completed in near future.
2 Methods
2.1 Data Acquisition
To construct the deformable Chinese human trunk atlas, 355 PET/CT images of healthy Chinese adults were collected from four large general hospitals located in northeast, southeast, and southwest of china. There are two reasons that we chose PET/CT images rather than MRI images: (1) most of the existing MRI in China focus on partial scanning rather than whole-body scanning (2) the construction of atlas need images of healthy subjects, these images can be acquired from PET/CT health screening.
From the PET/CT images collected, the age of subjects was unevenly distributed, the middle-aged were in the majority. Table 1 lists the number of subjects for each age group.
2.2 Images Segmentation and Registration
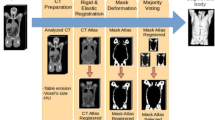
The first step of atlas construction is to segment three-dimensional trunk organs from the CT images. Due to the imperfect image quality of the low-dose CT images of PET/CT scan, we could only segment major organs with millimeter-level accuracy. The segmented organs include skin, muscle, subcutaneous fat, each individual bone, whole heart, lungs, liver, spleen, kidneys, and bladder. Among these organs, most of them were segmented manually except the skeleton and muscle using threshold method. The segmentation result was shown in Fig. 3b.
After the segmentation, all the segmented organs were converted to triangular surfaces meshes. The vertex correspondences between the training subjects were obtained by registering the organ meshes of the reference anatomy model using the point set registration method [16]. The reference anatomy model of whole body male and female anatomy were purchased from the web [17] and were cropped to create the trunk reference meshes. Figures 1 and 2 present the reference anatomy model of whole body and the trunk region. For the organs in trunk region which cannot be segmented properly, we used thin-plate-spline (TPS) transform method [18] to map these organs in the reference anatomy model based on the control points in nearby segmented organs.
2.3 Statistical Shape Modeling
Statistical shape modeling can model the inter-subject deformation of organ shapes. Coordinates for all n points are concatenated to one vector X that describes the shape of the ith subject:
The SSMs were based on a linear point distribution model described as:
where \( \varvec{X}\,{\mathbf{ \in }}\,\varvec{R}^{{{\mathbf{3}}\varvec{n}}} \) is a shape instance of the model, \( \bar{\varvec{X}}\,{\mathbf{ \in }}\,\varvec{R}^{{{\mathbf{3}}\varvec{n}}} \) is the mean value of the shape instances in the training set, \( \varvec{\varPhi}\,{\mathbf{ \in }}\,\varvec{R}^{{{\mathbf{3}}\varvec{N} \times \varvec{k}}} \) is the matrix of k shape modes, which are obtained using principal component analysis (PCA) of the mesh vertices and \( \varvec{b}\,{\mathbf{ \in }}\,\varvec{R}^{\varvec{k}} \) represents the shape coefficients that control the linear combination of variation modes.
2.4 Functional Metabolism Modeling
Besides the SSM, the volume of each organ was also calculated in order to evaluate the mean and variance of organ volumes of different sexes, ages and weights. The segmented regions of the CT images were mapped into the corresponding PET images to calculate the mean SUV of each organ (Fig. 3c). The SUV of PET image is defined as
where i is the voxel index, p i is the PET voxel value of voxel i, k is the coefficient to convert voxel value into radiation dose, C inject is the whole body injection dose of radioactive tracer, w i and w wb are the mass weights of voxel i and the entire body, respectively. SUV represents the ratio of tracer concentration in each voxel to the average concentration of unit body weight, it is commonly used in clinical environment to assess relative PET tracer concentration. In this study, the PET tracer is fluorodeoxyglucose (18F-FDG), which roughly reflects the absorption of glucose in human tissue. By calculating the mean SUV in each organ, we obtain organ-wise measurement of glucose metabolism level of each training subject.
3 Result
So far, we have segmented 138 CT images from the collected dataset, including 77 males and 61 females. Based on the segmented images, SSMs have been constructed for skin and muscles, major trunk organs and spine, respectively. At the current stage, the SSMs are built for all subjects including both sexes. Figures 4, 5 and 6 demonstrates the principal deformation modes of the SSMs.
Statistical shape models for major trunk organs, (c) the mean shape of training samples, (b) (d) and (a) (e) shows the variations of the largest and second largest deformation modes, respectively. The magnitudes of shape deformations are marked below each deformation result, where \( \varvec{\lambda}_{\varvec{i}} \) is the eigenvalue of the \( \varvec{i}^{{\varvec{th}}} \) deformation mode.
As revealed by Figs. 4 and 5, the largest inter-subject deformations (i.e. mode 1) are the changes of abdominal volume, which is affected by the amounts of subcutaneous fat and abdominal fat. The accumulation of fat tissue increases the thickness of subcutaneous fat (Fig. 5) and pushes the abdominal organs away from each other (Fig. 4). The second largest the inter-subject deformation is the motion of lungs and thoracic muscles caused by respiration. Such deformation is learned from the different breath holding levels of the training subjects. Figure 6 shows that inter-subject spine deformations mainly occur at upper abdominal vertebrae and lower cervical vertebrae.
Through the statistical analysis of organs volumes, we find that the mean volumes of male thoracic organs are 50–60% larger than females’, and the mean volumes of male abdominal organs are 30–40% larger than females’ (Fig. 7). Most of the organs volume increase with weight getting heavy, except for the lungs whose volumes are affected by inconsistent breath holding levels. The CT values (Fig. 8) in skeleton from both genders decrease after the age of 40, this finding coincided well with a previous study based on ultrasonic bone density tests of 8345 Chinese subjects [19]. The result also revealed that females have a significant decline of bone CT values after 40 years old, while the decline of male was not significant. The 18F-FDG SUVs (Fig. 9) of both genders also have a reduction after the age of 40, but the reduction of neither gender is significant.
4 Discussions and Conclusions
By now, the size of our sample set has reached a preliminary statistical significance, we are still collecting and segmenting more PET/CT images. The outcomes of shape modeling show the change rule of anatomical morphology of Chinese population samples. The statistical analysis of CT value and 18F-FDG SUVs reflects the change of skeleton density and metabolism from the subjects of different ages and sexes. However, this statistical result is still preliminary because our sample size is still limited. As the sample size keeps growing, more statistically meaningful results will be presented and dedicated atlases will be constructed for each gender. A publicly available website is under construction with the scope to share research results within one year.
References
Zhang, S., Jie, B., Tan, L.: The application of Chinese visible human dataset. FASEB J. 27, Ib8 (2013)
Sato, K., Noguchi, H., Emoto, Y., Koga, S., Saito, K.: Japanese adult male voxel phantom constructed on the basis of CT images. Radiat. Prot. Dosim 123, 337–344 (2007)
Saito, K., Wittmann, A., Koga, S., Ida, Y., Kamei, T., Funabiki, J., Zankl, M.: Construction of a computed tomographic phantom for a Japanese male adult and dose calculation system. Radiat. Environ. Bioph. 40, 69–75 (2001)
Nagaoka, T., Watanabe, S.: Postured voxel-based human models for electromagnetic dosimetry. Phys. Med. Biol. 53, 7047–7061 (2008)
Shi, C.Y., Xu, X.G., Stabin, M.G.: SAF values for internal photon emitters calculated for the RPI-P pregnant-female models using Monte Carlo methods. Med. Phys. 35, 3215–3224 (2008)
Lee, C., Lodwick, D., Hasenauer, D., Williams, J.L., Lee, C., Bolch, W.E.: Hybrid computational phantoms of the male and female newborn patient: NURBS-based whole-body models. Phys. Med. Biol. 52, 3309–3333 (2007)
Okada, T., Shimada, R., Hori, M., Nakamoto, M., Chen, Y.W., Nakamura, H., Sato, Y.: Automated segmentation of the liver from 3D CT images using probabilistic atlas and multilevel statistical shape model. Acad. Radiol. 15, 1390–1403 (2008)
Frangi, A.F., Rueckert, D., Schnabel, J.A., Niessen, W.J.: Automatic construction of multiple-object three-dimensional statistical shape models: application to cardiac modeling. IEEE Trans. Med. Imag. 21, 1151–1166 (2002)
Ellingsen, L.M., Chintalapani, G., Taylor, R.H., Prince, J.L.: Robust deformable image registration using prior shape information for atlas to patient registration. Comput. Med. Imaging Graph. 34, 79–90 (2009)
Baka, N., Kaptein, B.L., de Bruijne, M., van Walsum, T., Giphart, J.E., Niessen, W.J., Lelieveldt, B.P.: 2D-3D shape reconstruction of the distal femur from stereo X-ray imaging using statistical shape models. Med. Image Anal. 15, 840–850 (2011)
Benameur, S., Mignotte, M., Parent, S., Labelle, H., Skalli, W., de Guise, J.: 3D/2D registration and segmentation of scoliotic vertebrae using statistical models. Comput. Med. Imaging Graph. 27, 321–337 (2003)
Amunts, K., Lepage, C., Borgeat, L., Mohlberg, H., Dickscheid, T., Rousseau, M.É., Bludau, S., Bazin, P.L., Lewis, L.B., Orospeusquens, A.M.: BigBrain: an ultrahigh-resolution 3D human brain model. Science 340, 1472–1475 (2013)
www.itis.ethz.ch/itis-for-health/virtual-population/human-models/
Viceconti, M., Clapworthy, G., Taddei, F., Jan, S.V.S.: European virtual physiological human. J. Med. Biomed. 23, 19–25 (2008)
Boellaard, R., Krak, N.C., Hoekstra, O.S., Lammertsma, A.A.: Effects of noise, image resolution, and ROI definition on the accuracy of standard uptake values: a simulation study. J. Nucl. Med. 45, 1519–1527 (2004)
Jian, B., Vemuri, B.C.: A robust algorithm for point set registration using mixture of Gaussians. In: Tenth IEEE ICCV, vol. 1242, pp. 1246–1251 (2005)
www.turbosquid.com/3d-models/rigged-complete-male-female-3d-3ds/839850
Chui, H.L., Rangarajan, A.: A new point matching algorithm for non-rigid registration. Comput. Vis. Image Underst. 89, 114–141 (2003)
Wang, H.: Investigation of bone mineral density and osteoporosis prevalence in 8345 civil services from Shenzhen. China Mod. Dr. 49, 11–12 (2011)
Acknowledgement
This research was supported by the general program of National Natural Science Fund of China (Grant No. 61571076 and 81401475), the general program of Liaoning Science and Technology Project (Grant No. 2015020040), the cultivating program of Major National Natural Science Fund of China (Grant No. 91546123). The authors sincerely thank all the collaborating doctors for sharing the physical examination PET/CT images.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Wang, H., Sun, X., Huo, L., Tang, X., Liu, C. (2017). Construction of Deformable Trunk Atlas of Chinese Human Based on Multiple PET/CT Images: Preliminary Results. In: Duffy, V. (eds) Digital Human Modeling. Applications in Health, Safety, Ergonomics, and Risk Management: Ergonomics and Design. DHM 2017. Lecture Notes in Computer Science(), vol 10286. Springer, Cham. https://doi.org/10.1007/978-3-319-58463-8_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-58463-8_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58462-1
Online ISBN: 978-3-319-58463-8
eBook Packages: Computer ScienceComputer Science (R0)