Abstract
Brain correlates of cognitive performance have received considerable attention in the area of augmented cognition. Studies focused on the correlation between brain activations and cognitive load have laid their focus on connections integrated by frontal region. Most of the studies have manipulated visual or verbal cognitive load, though the effect of auditory memory load in cognitive performance is still unknown. In this study, functional near-infrared spectroscopy (fNIRS) of twelve subjects were measured when they were performing a paradigm of auditory working memory task. For the auditory n-back task, there are three experimental conditions, including two n-back task conditions of memorizing the stimuli with different memory load, and a condition of passive listening to the stimuli. The stimuli are sound combinations of major, minor, and dissonant chords. Hemodynamic responses from frontal brain regions were recorded using a wireless fNIRS device. Brain activations from ventrolateral and orbital prefrontal cortex are measured with signals filtered and baseline wandering removed. The fNIRS signals are then standardized with statistical test and group analysis carried out. The results revealed that there are significantly stronger hemodynamic responses in bilateral ventrolateral prefrontal cortex when subjects were attending to the auditory working memory task with high load. This study demonstrated the possibility of incorporating fNIRS as an index to evaluate cognitive performance regarding its benefit on the flexibility for portable applications than other neuroimaging techniques. The performance in cognitive function could therefore be quantitatively measured with the proposed method.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the past few years, functional near-infrared spectroscopy (fNIRS) is proved to be a flexible and convenient device to record brain hemodynamic response during the performance of cognitive tasks such as learning, memory, and motor reactions [1,2,3]. In several studies of memory function, the hemodynamic responses recorded by a fNIRS system from prefrontal cortex is claimed to be highly correlated with gray-matter functional magnetic resonance imaging (fMRI) activities during a working memory task [4]. Ogawa et al. have found that there is correlation between working memory performance and the neural activations measured using an fNIRS system. Subjects with better working memory performance have higher levels of oxyhemoglobin activations [5]. Consistent with the previous findings in fMRI, activations in lateral prefrontal cortex (LPFC) recorded from fNIRS systems are also proved to be associated with working memory in adults and even preschool children. The activations in the bilateral LPFC is depend on the memory-load [6]. These evidences suggest that fNIRS is useful and convenient for measuring the cognitive load and working memory performance [5].
Studies focused on the relationship between the brain activations and cognitive load have laid their focus on connections integrated by frontal region [7, 8]. Most of the studies have manipulated visual or verbal cognitive load, though the effect of auditory memory load in cognitive performance is still unknown. In this study, hemodynamic responses recorded from a fNIRS system of twelve subjects were measured when they were performing a paradigm of auditory n-back working memory task [9]. Brain activations from ventrolateral and orbital prefrontal cortex are measured with signals filtered and baseline wandering removed. This study demonstrated the possibility of incorporating fNIRS as an index to evaluate cognitive performance regarding its benefit on the flexibility for portable applications than other neuroimaging techniques.
2 Materials and Methods
2.1 NIRS Experiment and Preprocessing
The fNIRS signals are recorded using a wireless and portable system, BRAIN-NIRS Hb13 (ASTEM Co. Ltd., Japan), as shown in Fig. 1A. The concentration of oxygenated hemoglobin (oxy-Hb) and deoxygenated hemoglobin (deoxy-Hb) are recorded from four locations of the scalp. The center of the probe is placed in the frontal area (Fpz), and four sensors were set on Fp1, Fp2, F7, and F8 according to the international 10–20 system for electroencephalography, as illustrated in Fig. 1B [10]. These four positions are corresponded to the left/right dorsal and ventral prefrontal cortex (DLPFC and VLPFC), respectively, based on an anatomical cranio-cerebral correlation study [11, 12]. The concentration change of oxy-Hb is used for further analysis since it is more sensitive to the changes of cerebral blood flow.
Twelve subjects were recruited in the experiment with their fNIRS data recorded in a shielded room. The raw data are band-pass filtered (0.01–0.1 Hz) to attenuate the high frequency noise, respiration, and cardiac cycle effects [13,14,15]. The data recorded from each subject are checked for any potential saturation when light intensity at the detector was higher than the device limit. The signals are then standardized with baseline-wandering removed. Group analysis and statistical test are then carried out to compare different conditions of working memory load.
2.2 Auditory N-Back Working Memory Task
The subjects are requested to participate in a paradigm of auditory n-back working memory task [9]. The memory load are manipulated in this auditory task with different type of emotional stimuli. There are three conditions with distinct memory load during chord listening: 1-back (1B), 2-back (2B), and a task of passive listening (PL) to the stimuli. Each music stimulation is composed of four sound combinations of one of the major, minor, or dissonant chords. A random combination of task conditions (PL, 1B or 2B) and chord categories (major, minor or dissonant) is designed as a stimulation in each trial. Each participant are requested to attend a 2-session experiment with a 2-min rest with each session consists of 18 blocks. Twenty trials were presented in a block with each trial constructed of a sound lasting 1000 ms, followed by a 1500-ms silence before the next trial. Participants were instructed to press the left button in the n-back task when they recognized the chord matching that of the last n trials.
3 Results and Discussions
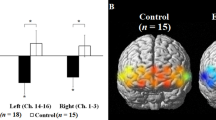
The behavior results in the auditory n-back task revealed that the average correctness of the 1-back task is 85.6 \( \pm \) 7.1%, which is 17.8% larger than that of the 2-back task (67.8 \( \pm \) 7.5%). As illustrated in Fig. 2, the hemodynamic responses recorded by the fNIRS system are standardized to z-score. Stronger activations were observed from channel 1 and 4, which are localized over VLPFC. Activations are more pronounced in higher working memory load. In left VLPFC (channel 1), significant difference (p = 0.01) was found between PL and 1B. The difference is more pronounced in right VLPFC with significance between PL and 1B (p = 0.006), and also PL and 2B (p = 0.004). The results revealed that there are significantly stronger hemodynamic responses in bilateral VLPFC when subjects were attending to the auditory working memory task with higher memory load. The findings in this study showed consistent results with previous studies in visual working memory study [6]. The cognitive performance could therefore be quantitatively and consistently measured.
Hemodynamic responses recorded by the fNIRS system when subjects were attending to n-back auditory working memory tasks including three conditions: passive listening (PL), one-back (1B), and two-back (2B). Stronger activations were observed from channel 1 and 4, which are localized over ventrolateral prefrontal cortex. Activations were more pronounced in higher working memory load.
4 Conclusions
This study demonstrated the flexibility of incorporating fNIRS as an index to evaluate cognitive performance. In addition, fNIRS can potentially be applied to functional mapping in childhood or patients with mental disorder [6]. Since it imposes fewer constraints on behavior than fMRI, fNIRS appears to be more practical than fMRI for investigating cognitive neuroscience on the primate cortex [16]. In addition to the studies of brain functions, fNIRS may also be a useful tool to the development of brain-computer interface [17,18,19] or the validation of drugs for mental diseases that can cause reduction in lateral prefrontal activities accompanied by improved cognitive performance [20].
References
Noah, J.A., Ono, Y., et al.: fMRI validation of fNIRS measurements during a naturalistic task. JoVE - J. Visualized Exp. (2015). doi:10.3791/52116
Shimada, S., Hiraki, K.: Infant’s brain responses to live and televised action. NeuroImage 32(2), 930–939 (2006)
Shimada, S., Oki, K.: Modulation of motor area activity during observation of unnatural body movements. Brain Cogn. 80(1), 1–6 (2012)
Sato, H., Yahata, N., et al.: A NIRS–fMRI investigation of prefrontal cortex activity during a working memory task. NeuroImage 83, 158–173 (2013)
Ogawa, Y., Kotani, K., Jimbo, Y.: Relationship between working memory performance and neural activation measured using near-infrared spectroscopy. Brain Behav. 4(4), 544–551 (2014)
Tsujimoto, S., Yamamoto, T., et al.: Prefrontal cortical activation associated with working memory in adults and preschool children: an event-related optical topography study. Cereb. Cortex 14(7), 703–712 (2004)
Kane, M.J., Engle, R.W.: The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon. Bull. Rev. 9(4), 637–671 (2002)
Ma, L., Steinberg, J.L., et al.: Working memory load modulation of parieto-frontal connections: evidence from dynamic causal modeling. Hum. Brain Mapp. 33(8), 1850–1867 (2012)
Pallesen, K.J., Brattico, E., et al.: Cognitive control in auditory working memory is enhanced in musicians. PLoS ONE 5(6), e11120 (2010)
Jasper, H.H.: The ten twenty electrode system of the international federation. Electroencephalogr. Clin. Neurophysiol. 10, 371–375 (1958)
Okamoto, M., Dan, H., et al.: Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage 21(1), 99–111 (2004)
Sanefuji, M., Takada, Y., et al.: Strategy in short-term memory for pictures in childhood: a near-infrared spectroscopy study. Neuroimage 54(3), 2394–2400 (2011)
Izzetoglu, M., Izzetoglu, K., et al.: Functional near-infrared neuroimaging. IEEE Trans. Neural Syst. Rehabil. Eng. 13(2), 153–159 (2005)
Ayaz, H., Izzetoglu, M., et al.: Sliding-window motion artifact rejection for functional near-infrared spectroscopy. In: Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (2010)
McKendrick, R., Ayaz, H., et al.: Enhancing dual-task performance with verbal and spatial working memory training: continuous monitoring of cerebral hemodynamics with NIRS. NeuroImage 85(3), 1014–1026 (2014)
Fuster, J., Guiou, M., et al.: Near-infrared spectroscopy (NIRS) in cognitive neuroscience of the primate brain. Neuroimage 26(1), 215–220 (2005)
Coyle, S., Ward, T., et al.: On the suitability of near-infrared (NIR) systems for next-generation brain–computer interfaces. Physiol. Meas. 25(4), 815 (2004)
Fazli, S., Mehnert, J., et al.: Enhanced performance by a hybrid NIRS–EEG brain computer interface. Neuroimage 59(1), 519–529 (2012)
Kaiser, V., Bauernfeind, G., et al.: Cortical effects of user training in a motor imagery based brain–computer interface measured by fNIRS and EEG. Neuroimage 85, 432–444 (2014)
Ramasubbu, R., Singh, H., et al.: Methylphenidate-mediated reduction in prefrontal hemodynamic responses to working memory task: a functional near-infrared spectroscopy study. Hum. Psychopharmacol. Clin. Exp. 27(6), 615–621 (2012)
Acknowledgment
This work was supported by grant 105-2221-E-030-001- from the Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this paper
Cite this paper
Wu, SM., Ding, HM., Tseng, YL. (2017). A Functional Near-Infrared Spectroscopy Study of Auditory Working Memory Load. In: Stephanidis, C. (eds) HCI International 2017 – Posters' Extended Abstracts. HCI 2017. Communications in Computer and Information Science, vol 713. Springer, Cham. https://doi.org/10.1007/978-3-319-58750-9_38
Download citation
DOI: https://doi.org/10.1007/978-3-319-58750-9_38
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-58749-3
Online ISBN: 978-3-319-58750-9
eBook Packages: Computer ScienceComputer Science (R0)