Abstract
A percutaneous electrogastrogram (EGG) is a simple and low-restraint way to measure the electrical activity of the gastrointestinal tract. An electrogastrogram examination is a noninvasive method of evaluating gastrointestinal motility and autonomic nervous system activity. However, EGGs are not as widely used in clinical settings as electrocardiograms (ECGs) or electroencephalographs (EEGs) because an EGG can be impacted by electrical activity from the myocardium and diaphragm (due to respiration), and there is no method to relate the functions of the stomach to the data obtained. This paper examines the effect of exercise on gastric electrical activity using two exercise intensities to confirm the basic biological response of an EGG. It was found that after high-intensity exercising the spectrum density at the normal frequency band of the stomach (2.4–3.7 cpm) decreased, which may indicate a decline in gastric activity during exercise. Exercise intensity is thought to affect the electrical activity of not only the gastrointestinal tract but also other organs.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
The intestines, which are referred to as “the second brain,” are part of the digestive tract, and are linked to the brain through the autonomous nervous system and intercellular messages, such as hormones and cytokine [1]. Abdominal pain, diarrhea, constipation and other digestive issues caused by stress are a result of disrupted balance of the autonomic nerves. The intestines not only digest food but also immunize the body, and are linked to cancer and diabetes [2]. In addition, a part of the intestines produces a precursor of serotonin, which not only maintains a person’s overall physical health but also helps stabilize the heart [3]. Therefore, maintaining healthy intestines contributes to mental and physical health. Recently, intestinal floral boom has attracted interest in the fields of intestinal environment and health. In the intestines, probiotic bacteria, pathogenic bacteria, and opportunistic bacteria coexist and protect the intestinal environment. To regulate the intestinal environment, people must eat carefully, exercise, and rest. Exercise promotes circulation and keeps the autonomous nervous system healthy. Maintaining the autonomous nerves in this way can encouraging peristaltic movement of the intestines [4, 5]. Excessive exercise, however, results in sympathetic nerve dominance, which suppresses digestive tract functions. Therefore, it is important to use appropriate exercise intensity.
The percutaneous electrogastrogram (EGG), which can perform noninvasive, low-restraint measurements of the electric activity that controls gastrointestinal motility, is one way to examine the motor functions of gastrointestinal disorders [6, 7]. Regular electrical activity, such as that of the heart, is also observed in the stomach and intestines, where repeated depolarization and repolarization occur. The pacemaker of gastric electrical activity exists in the upper one-third section of the abdomen, where it transmits electrical waves to the pyloric region at a rate of 3 cycles per minute (cpm). This pacemaker is controlled by parasympathetic nervous system activity; however, the cyclic electrical activity occurs spontaneously, and is stimulated by a network of islands called “interstitial cells of Cajal” (ICCs) [8,9,10,11]. Peristaltic movement does not occur when electrical signals are emitted from the ICCs. However, when the contraction threshold is exceeded during depolarization, an action potential is produced that causes peristaltic movement. This electrical activity is divided into two parts: electrical response activity (ERA), which is accompanied by a peristaltic movement; and electrical control activity (ECA), which is not accompanied by the peristaltic movement [12]. Because an EGG cannot distinguish these, it cannot directly measure peristaltic movements [13]. However, it is thought that EGGs are linked to gastric electrical activity [14], and that it is possible to discover abnormal peristaltic movement using a response confirmation test. This study examines the impact of exercise intensity on gastric electrical activity by a participant undergoing an EGG during exercising.
2 Experimental Method
The experiment participants were 19 young men aged 22–27 (average ± standard deviation: 22.8 ± 1.4) years, who had no history of digestive disorders or symptoms. The experiment was fully explained to the subjects prior to the experiment, and they agreed to participate after reading a document describing the purpose and significance of the study, as well as the policies outlining the protection of their privacy, handling of data, and guarantee of interruption. The electronic data obtained during the experiment was recorded under conditions of untraceable anonymity, and approval for the experiment (H2017002) was granted by the ethics committee of the Graduate School of Engineering, University of Fukui.
EGGs and electrocardiograms (ECGs) were performed between 60 min of rest in a supine position before and after the exercise. The participants ran at a velocity of 10 km/h (high-intensity exercise) and walked at 5 km/h (intermediate-intensity exercise) for 15 min each on a treadmill (DK-822E, DAIKOU). A control experiment was conducted by having the participants stand still for 15 min (low-intensity exercise). The MET scores of the exercises were approximately 9–10, 3–4, and 1.0–1.5 (which is within the range of everyday exercise) for the high-intensity, intermediate-intensity [15], and low-intensity exercises, respectively. Each measurement was taken on a different day, and on each day, the patient sequence was randomized to reduce the effect of ordering.
The EGGs were obtained using an ECG disposal electrode (Blue Sensor, Mets Inc.), as shown in Fig. 1. In this study, the EGG electrode was affixed to two places near the stomach pacemaker (ch 1) and the pyloric part (ch 2). In addition, the ECGs that were recorded simultaneously with the EGGs were recorded by II induction. The electrode was attached to the skin after ethanol was used to disinfect and lower skin resistance. The EGG was recorded using bipolar leads, amplification was performed using a biological amplifier (Biotop mini, East Medic Co., Ltd.), and data were recorded on a PC with an analog input card (ADA16-32/2(CB)F, CONTEC). The following settings were used for the biological amplifier: sensitivity = 100 μV, low frequency cut-off filter = 0.02 Hz, and high-frequency cut-off filter = 0.5 Hz. Participants were asked to eat 400 kcal of portable food (Calorie-Mate, Otsuka Pharmaceutical Co., Ltd.) two hours before the start of the experiment, but nothing afterwards in order to equalize the time food had remained in the stomachs of all the subjects.
3 Analytical Method
The EGGs and ECGs that were recorded were A/D converted at 1 kHz to obtain timeseries data. A bandpass filter with cut-off frequencies 0.015–0.15 Hz was applied to the data that had been obtained to remove mixed electromyograms (EMGs) and electrical noise due to the EGG time series equipment. The 1-kHz EGG time series was resampled at 10 Hz because the normal cycle of an EGG is relatively slow (about 3 cpm). In this study, the time series was analyzed using running spectrum analysis. The EGG time series were moved and the 8,192-point time windows (roughly 13 min) were divided into intervals of 3000 points (5 min) before each was analyzed. The analyzed sections were recorded (as explained below) with the start times representing the analyzed sections.
Frequency analysis was carried out by performing a Fourier conversion on each time series section, focusing on bradygastria (1.1–2.4 cpm), reference frequency band (2.4–3.7 cpm), and tachygastria (3.7–5.0 cpm) [18]. The power spectral density (PSD) in these frequency bands was also calculated using 6.0–8.0 cpm, because a study showed that a fluctuation of approximately 7 cpm in an EGG reflects the electrical activity of the colon [19].
The EGG time series sections were also analyzed using a statistically estimated translational error based on a Wayland algorithm [16, 17]. Here, the translational error (Etrans) estimated based on the Wayland algorithm is an index that quantitively evaluates the smoothness of the course of the attractor buried in the phase space. If the trajectory of the attractor reconstituted in the embedded space is smooth, it is said that the time series is deterministic. If the translational error is a positive, near-zero value, and the model that constitutes the time series is deterministic and large, the error can be viewed as probabilistic. When an object can be modelled using Brownian movement, the translational error value is estimated to be 1.
The ECG measured alongside the EGG was analyzed using heart rate variability (HRV) analysis [20], which can quantify the indices of the sympathetic and parasympathetic nervous systems by analyzing the RR interval (heart rate) from the time and frequency domains. The RR interval time series was abstracted, moved, and divided into 512 time windows at 5 min intervals, which were analyzed such that they corresponded to the sections of the EGG time series. This study assumed the low frequency/high frequency (LF/HF) ratio and heart rate (HR) to be the activity indices of the sympathetic nerves; also, the PSD of the LF constituent is assumed to be 0.04–0.15 Hz, and that of the HF constituent 0.15–0.4 Hz.
For the calculated analysis indices, the average values recorded for each time before and after exercise were compared using the Wilcoxon signed-rank test (at a significance level of 0.05).
4 Results
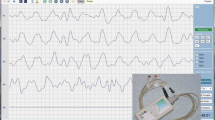
Figure 2 shows the EGG waves corresponding to 10–15 min after the start of measurement for each exercise. From the visual observation in ch 2, compared to that in ch 1, the effect of the fine waves derived from the intestinal tract is large. The EGG waves after a low-intensity exercise show a large period of approximately 3 cpm. However, in the EGG wave, after a high-intensity exercise, the amplitude decreased from that of the pre-exercise level and its period approximately 3 cpm also reduced in scale.
High-speed Fourier conversions of the EGG time series sections were completed to calculate the PSD for each band—bradygastria (1.1–2.4 cpm), reference frequency (2.4–3.7 cpm), tachygastria (3.7–5.0 cpm), and the colon (6.0–8.0 cpm); the results are shown in Figs. 3, 4, 5 and 6. For low- and intermediate-intensity exercises, consistently significant differences were not seen in any bands. For the high-intensity exercise, the values in the reference frequency band were significantly lower after exercise than before (Fig. 5b).This trend was sustained for 35 min from the start of post-exercise measurements, and the values in the bradygastria band were significantly higher after the exercise than before the exercise was performed (Fig. 5a). This significant trend was sustained over 35–45 min.
The ECG recorded alongside the EGG was analyzed. For a low-intensity exercise, the heart rate increased significantly from pre-exercise (at 0 min) to 10 min post-exercise (Fig. 7a). For intermediate-intensity exercise, the heart rate increased significantly from pre-exercise to 30 min post-exercise (Fig. 7b). For a high-intensity exercise, the heart rate increased significantly from pre-exercise to 45 min post-exercise (Fig. 7c). The LF/HF for an intermediate-intensity exercise increased significantly in some cases from pre-exercise to 45 min post-exercise (Fig. 8b). The LF/HF for high intensity exercise increased significantly from pre-exercise to 45 min post-exercise (Fig. 8c).
5 Discussion
In this study, EGGs were used to measure gastric electrical activity during exercising to study the influence of exercise intensity on gastric electrical activity. The results showed that for a high intensity exercise, the PSD in the gastric normal frequency band tends to decrease, presumably indicating a temporary decline of gastric activity caused by the exercise. This reveals the possibility that digestive activity decreases after a high-intensity exercise. For low- and intermediate-intensity exercises, no consistently significant differences were seen in any bands.
For this reason, for an exercise with a MET score 1.0–4.0, the influences of the EGGs on each frequency band were small, and it was confirmed that the gastric electrical activity was constant at this time. For a high-intensity exercise (e.g., running), the PSD of the gastric normal frequency band decreased. This could indicate a temporary decline of gastrointestinal activity caused by the exercise. For a high-intensity exercise, a remarkable rise in PSD was seen in the bradygastria band after the exercise. Bradygastria shows the integration power of the slow wave domain of the power spectrum; thus, it is possible that it caused the delay and reduction of gastric emptying ability after running.
Generally, under the effects of the autonomic nerve balance, parasympathetic nerve activity is temporarily dominant, and the digestive tract activity is aggravated; additionally, if sympathetic nerve activity is dominant, the digestive tract activity is repressed. The phenomena revealed in this study are presumed to be the results of exercise activity that make the sympathetic nerve activity dominant and repress the digestive tract activity. Clarifying to what extent this temporary parasympathetic nerve activity and repression of digestive tract activity continue and how they affect the later digestive tract activity would be extremely significant from the perspective of hygiene. It is assumed, however, that a social-intensive environment, an irregular lifestyle that ignores circadian rhythm, a change of environment, excessive stress, and other such conditions disrupt the autonomic nerve balance, thereby causing autonomic imbalance. This is known to cause functional gastrointestinal disorders. In this study, a high-intensity exercise is understood to stimulate the regulation ability of the autonomic nerve balance.
6 Conclusion
This paper reports the use of EGGs to measure gastric electrical activity during exercise to study the influence of exercise intensity on gastric electrical activity. The results show that it is possible for a high-intensity exercise to be followed by the temporary inducement of a state unsuitable for food digestion. In the future, following multi-faceted discussions of the complexity of generators of EGGs, we will study the use of wearable devices to measure gastrointestinal electrical activity to prepare life logs.
References
Fukudo, S., Nomura, T., Hongo, M.: Impact of corticotropin-releasing hormone on gastrointestinal motility and adrenocorticotropic hormone in normal controls and patients with irritable bowel syndrome. Gut 42(6), 845–849 (1998)
Miyamura, M.: New Exercise Physiology Publication Department of Medical Books, vol. 2. Shinko Trading Co., Ltd (2001)
Gǔ, L., Fukudo, S.: Irritable bowel disorder and serotonin. Psychosom. Med. 50(1), 11–17 (2010)
Peters, H., de Vries, W.R., Vanberge-Henegouwen, G.P., Akkermans, L.M.: Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut 48(3), 435–439 (2001)
Verger, P., Lanteaume, M.T., Louis-Sylvestre, J.: Human intake and choice of foods at intervals after exercise. Appetite 18(2), 93–99 (1992)
Alvarez, W.C.: The electrogastrogram and what is shows. J. Am. Med. Assoc. 78, 1116–1119 (1922)
Kenneth, L.K., Robert, M.: Handbook of Electrogastrography. Oxford University Press, Oxford (2004)
Nakamura, E., Kito, Y., Fukuda, H., Yanai, Y., Hashitani, H., Yamamoto, Y., Suzuki, H.: Cellular mechanism of spontaneous activity of the stomach smooth muscle. Nihon Yakurigaku Zasshi 123(3), 141–148 (2002)
Torihashi, S.: Structure and functions of the Cajal cells. Pediatr. Surg. 37(4), 467–472 (2005)
Takayama, I., Horiguchi, K., Daigo, Y., Mine, T., Fujino, M.A., Ohno, S.: The interstitial cells of Cajal and a gastroenteric pacemaker system. Arch. Histol. Cytol. 65(1), 1–26 (2002)
Thomsen, L., Robinson, T.L., Lee, J.C.F., Farraway, L.A., Hughes, M.J., Andrews, D.W., Huizinga, J.D.: Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 4, 848–851 (1998)
Smout, A.J.P.M., Van Der Schee, E.J., Grashuis, J.L.: What is measured in electrogastrography? Dig. Dis. Sci. 25(3), 179–187 (1980)
Chen, J.Z., McCallum, R.W.: Electrogastrography: Principles and Applications. Raven Press, New York (1994)
Pezzolla, F., Riezzo, G., Maselli, M.A.: Electrical activity recorded from abdominal surface after gastrectomy or colectomy in humans. Gastroenterology 97(2), 313–320 (1989)
Ainsworth, B.E., Haskell, W.L., Herrmann, S.D., Meckes, N., Bassett Jr., D.R., Tudor-Locke, C., Greer, J.L., Vezina, J., Whitt-Glover, M.C., Leon, A.S.: Compendium of physical activities: a second update of codes and MET values. Med. Sci. Sports Exerc. 43(8), 1575–1581 (2011)
Wayland, R., Bromley, D., Pickett, D., Passamante, A.: Recognizing determinism in a time series. Phys. Rev. Lett. 70(5), 580–582 (1993)
Takada, H., Simizu, Y., Hoshita, H., Shiozawa, T.: Wayland tests for differenced time series could evaluate degrees of visible determinism. Bull. Soc. Sci. Form 19(3), 301–310 (2005)
Japan Society of Neurovegetative Research: Autonomic nerve function examination. vol. 5. Bunkodo Co., Ltd. (2007)
Homma, S.: Isopower mapping of the electrogastrogram (EGG). J. Auton. Nerv. Syst. 62(3), 163–166 (1997)
Pomeranz, B., Macaulay, R.J., Caudill, M.A., Kutz, I., Adam, D., Gordon, D., Kilborn, K.M., Barger, A.C., Shannon, D.C., Cohen, R.J., Benson, H.: Assessment of autonomic function in humans by heart rate spectral analysis. Am. J. Physiol. 248, 151–153 (1985)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this paper
Cite this paper
Kinoshita, F., Fujita, K., Miyanaga, K., Touyama, H., Takada, M., Takada, H. (2018). Analysis of Electrogastrograms During Exercise Loads. In: Antona, M., Stephanidis, C. (eds) Universal Access in Human-Computer Interaction. Virtual, Augmented, and Intelligent Environments . UAHCI 2018. Lecture Notes in Computer Science(), vol 10908. Springer, Cham. https://doi.org/10.1007/978-3-319-92052-8_22
Download citation
DOI: https://doi.org/10.1007/978-3-319-92052-8_22
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-92051-1
Online ISBN: 978-3-319-92052-8
eBook Packages: Computer ScienceComputer Science (R0)