Summary
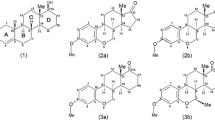
The effect of the substitution in position 1 on the low-energy conformations of the oxytocin/vasopressin 20-membered ring was investigated by means of molecular mechanics. Three representative substitutions were considered: β′-mercapto-β,β-dimethyl)propionic acid (Dmp), (β′-mercapto-β,β-cyclopentamethylene)propionic acid (Cpp), both forming strong antagonists, and (α,α-dimethyl-β-mercapto)propionic acid (α-Dmp), forming analogs of strongly reduced biological activity, with the β-mercaptopropionic (Mpa) residue taken as reference. Both ECEPP/2 (rigid valence geometry) and AMBER (flexible valence geometry) force fields were employed in the calculations. Three basic types of backbone conformations were taken into account which are distinguished by the type of β-turn at residues 3 and 4: β1/βIII, βII, and βI′/βIII′, all types containing one or two intra-annular hydrogen bonds. The allowed (ring-closed) disulfide-bridge conformations were searched by an algorithm formulated in terms of scanning the disulfide-bridge torsional angle Cβ-S-S-Cβ. The ECEPP/2 and AMBER energies of the obtained conformations were found to be in reasonable agreement. Two of the low-energy conformers of the [Mpa1]-compound agreed very well with the cyclic part of the two conformers found in the crystal structure of [Mpa1]-oxytocin. An analysis of the effect of β-substitution on relative energies showed that the conformations with the N-C′-CH2-CH2 (ψ′1) and C′-CH2-CH2-S (ϰ′1) angles of the first residue around (−100°, 60°) and (100°, −60°) are not affected; this in most cases implies a left-handed disulfide bridge. In the case of α-substitution the allowed values of ψ′1 are close to ± 60°. This requirement, being in contradiction to the one concerning β-substitution, could explain the very low biological activity of the α-substituted analogs. The conformational preferences of substituted compounds can largely be explained by the analysis of local interactions within the first residue. Based on the selection of the conformations which are low in energy for both the reference and β-substituted compounds, two distinct types of possible binding conformations were proposed, the first one being similar to the crystal conformer with a left-handed disulfide bridge, the second one having a right-handed bridge, but a geometry different from that of the crystal conformer with the right-handed bridge. The first type of disulfide-bridge arrangement is equally favorable for both βI/βIII and βII types of backbone structure, while the second one is allowed only for the βII type of backbone. No conformation of the βI′/βIII′ type has a low enough energy to be considered as a possible binding conformation for all of the active compounds studied in this work.
Similar content being viewed by others
References
Gazis, D., In Jošt, K., Lebl, M. and Brtnik, F. (Eds.) CRC Handbook of Neurohypophyseal Hormone Analogs, Vol. 1, Part 1, CRC Press, Boca Raton, FL, 1987, pp. 106–107.
Hruby, V.J. and Lebl, M., In Jošt, K., Lebl, M. and Brtnik, F. (Eds.) CRC Handbook of Neurohypophyseal Hormone Analogs, Vol. 1, Part 2, CRC Press, Boca Raton, FL, 1987, pp. 152–155.
Shenderovich, M.D., Kasprzykowski, F., Liwo, A., Sekacis, I., Saulitis, J. and Nikiforovich, G.V., Int. J. Pept. Protein Res., 38 (1991) 528.
Tarnowska, M., Liwo, A., Grzonka, Z. and Tempczyk, A., In Kuchař, M. (Ed.) QSAR in Design of Bioactive Compounds, Vol. 2, J.R. Prous Science Publishers, Barcelona, 1991, pp. 361–436.
Momany, F.A., McGuire, R.F., Burgess, A.W. and Scheraga, H.A., J. Phys. Chem., 79 (1975) 2361.
Némethy, G., Pottle, M.S. and Scheraga, H.A., J. Phys. Chem., 87 (1983) 1883.
Weiner, S.J., Kollman, P.A., David, A.C., Chandra Singh, U., Ghio, C., Alagona, G., Profeta, S. and Weiner, P., J. Am. Chem. Soc., 106 (1984) 765.
Roterman, I.K., Lambert, M.H., Gibson, K.D. and Scheraga, H.A., J. Biomol. Struct. Dyn., 7 (1989) 421.
Palmer, K.A. and Scheraga, H.A., J. Comput. Chem., 12 (1990) 505.
Liwo, A., Tempczyk, A. and Grzonka, Z., J. Comput-Aided Mol. Design, 2 (1988) 281.
Nikiforovich, G.V., Leonova, V.I., Galaktionov, S.G. and Chipens, G.I., Int. J. Pept. Protein Res., 13 (1979) 363.
Spasov, V.Z. and Popov, E.M., Soviet J. Bioorg. Chem., 7 (1981) 263.
Dunfield, L.G., Burgess, A.W. and Scheraga, H.A., J. Phys. Chem., 82 (1978) 2609.
Gō, N. and Scheraga, H.A., Macromolecules, 3 (1970) 178.
Bruccoleri, R.E. and Karplus, M., Macromolecules, 18 (1985) 2767.
Bruccoleri, R.E. and Karplus, M., Science, 235 (1987) 458.
Palmer, K.A. and Scheraga, H.A., J. Comput. Chem., 13 (1991) 329.
Ortega, J.M. and Rheinboldt, W.C., Iterative Solution of Nonlinear Equations in Several Variables, Academic Press, New York, NY, 1970, pp. 183–187.
Bhaskaran, R., Chuang, L.-C. and Yu, C., Biopolymers, 32 (1992) 1599.
Scheraga, H.A., Biopolymers, 20 (1981) 1877.
Wood, S.P., Tickle, I.J., Trehorne, A.M., Prits, J.E., Mascarenhas, Y., Li, J.Y., Husoin, J., Cooper, S., Blundell, T.L., Hruby, V.J., Buken, A., Sishman, A.J. and Wyssbrod, H.P., Science, 232 (1986) 633.
Prasad, B.V.V. and Balaram, P., CRC Crit. Rev. Biochem., 16 (1984) 307.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tarnowska, M., Liwo, A., Shenderovich, M.D. et al. A molecular mechanics study of the effect of substitution in position 1 on the conformational space of the oxytocin/vasopressin ring. J Computer-Aided Mol Des 7, 699–719 (1993). https://doi.org/10.1007/BF00125327
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00125327