Abstract
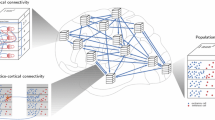
A method for modeling anatomical connectivity for a vertically organized slab of cortical tissue in mammalian primary visual cortex has been developed. The modeled slab covers 500 × 500 μm of cortical surface and extends vertically throughout the full depth of the cortex. The model slab was divided into 6 laminae and neuronal somata were distributed in three dimensions through the slab in accordance with experimentally derived cell densities. Axonal and dendritic arborizations were modeled as line segments. A total of 17 morphological types of neurons were included. Connectivity was established based on proximity between axonal and dendritic arbors. There is good general agreement between the vertical distribution of connections generated by the model and the vertical distribution of synapses observed for cat area 17. In all layers, fewer connections were generated in the model than synapses in cat area 17. This is due, at least in part, to the exclusion of long range intracortical projections and sources of afferent input other than the dorsal lateral geniculate nucleus from the model. The connection scheme described here will be used in conjunction with a physiology model to model vertical signal flow, and will be expanded further to model receptive fields of cortical neurons.
Similar content being viewed by others
References
Beaulieu C, Colonnier M (1983) The number of neurons in the different laminae of the binocular and monocular regions of area 17 in the cat. J Comp Neurol 217:337–344
Beaulieu C, Colonnier M (1985) A laminar analysis of round-asymmetrical and flat-asymmetrical synapses on spines, dendritic trunks and cell bodies in area 17 of the cat. J Comp Neurol 231:180–189
Chagnac-Amitai Y, Connors BW (1989) Horizontal spread of synchronized activity in neocortex and its control by GABA mediated inhibition. J Neurophysiol 425:1271–1281
Colonnier M (1981) The electron microscopic analysis of the neuronal organization of the cerebral cortex in the organization of the cerebral cortex: Schmitt FO, Worden FG, Adelman G, Dennis SG (eds) Proceedings of a Neurosciences Research Program Colloquium. MIT Press, Cambridge, Mass
Crick F, Asanuma C (1987) In: McClelland JL, Rumelhart D, the PDP Research Group (eds) Certain aspects of the anatomy and physiology of the cerebral cortex in parallel distributed processing: explorations in the microstructure of cognition vol 2: Psychological and biological models. MIT Press, Cambridge, Mass, pp 333–371
Cunningham ET, LeVay S (1986) Laminar and synaptic organization of the projection from the thalamic nucleus centralis to primary visual cortex in the cat. J Comp Neurol 254:65–77
Douglas RJ, Martin KAC (1990) In: Shepherd GM (ed) Neocortex in the synaptic organization of the brain 3rd edn. Oxford University Press, Oxford, pp 389–438
Fleischhauer K (1974) On the different patterns of dendritic bundling in the cerebral cortex of the cat. Z Anat Entwickl Gesch 143:115–126
Freeman WJ (1979a) Non-linear dynamics of palecortex manifested in olfactory EEG. Biol Cybern 35:221–234
Freeman WJ (1979b) EEG analysis gives model of neuronal template matching mechanism for sensory search in olfactory bulb. Biol Cybern 35:221–234
Freund TF, Martin KAC, Smith AD, Somogyi P (1983) Glutamate decarboxylase-immunoreactive terminals of Golgi-impregnated axoaxonic cells and of presumed basket cells in synaptic contacts with pyramidal neurons of the cat's visual cortex. J Comp Neurol 221:263–278
Friedlander MJ, Lin CS, Stanford LR, Sherman SM (1981) Morphology of functionally identified neurons in the lateral geniculate nucleus of the cat. J Neurophysiol 46:80–129
Gabbott PA, Somogyi P (1986) Quantitative distribution of GABA immunoreactive neurons in the visual cortex (area 17) of the cat. Brain Res 61:323–331
Gilbert CD (1983) Microcircuitry of the visual cortex. Ann Rev Neurosci 6:217–247
Gilbert CD, Wiesel T (1983) Clustered intrinsic connections in cat visual cortex. J Neurosci 3:1116–1133
Houk JC (1990) In: Selverson AI (ed) Modeling the cerebellum in neural computation. Society of Neuroscience, Washington D.C.
Humphrey AL, Sur M, Uhlrich DJ, Sherman SM (1985a) Projection patterns of individual x and y cell axons from the lateral geniculate nucleus to cortical area 17 in the cat. J Comp Neurol 233:159–189
Humphrey AL, Sur M, Uhlrich DJ, Sherman SM (1985b) Termination patterns of individual x and y cell axons in the visual cortex of the cat: projections to area 18, to the 17/18 border region, and to both areas 17 and 18. J Comp Neurol 233:190–212
Kisvarday ZF, Martin KAC, Somogyi P, Whitteridge D (1983) The physiology, morphology, and synaptology of basket cells in the cat's visual cortex. J Physiol (London) 334:21–22P
LeVay S (1986) Synaptic organization of claustral and geniculate afferents to the visual cortex of the cat. J Neurosci 6:3564–3575
LeVay S, Ferster D (1979) Proportion of interneurons in the cat's lateral geniculate nucleus. Brain Res 164:304–308
Li Z (1990) A model of olfactory adaptation and sensitivity enhancement in the olfactory bulb. Biol Cybern 62:379–392
Lund JS, Henry GH, MacQueen CL, Harvey AR (1979) Anatomical organization of the primary visual cortex (area 17) of the cat: a comparison with area 17 of the macaque monkey. J Comp Neurol 184:599–617
Martin KAC, (1988) From single cells to simple circuits in the cerebral cortex. Q J Exp Physiol 73:637–702
Mitzdorf U, Singer W (1978) Prominent excitatory pathways in the cat visual cortex (A 17 and A 18): a current source density analysis of electrically evoked potentials. Exp Brain Res 33:371–394
Mitchison G, Crick F (1982) Long axons within the striate cortex: their distribution, orientation, and patterns of connection. Proc Natl Acad Sci USA 79:3661–3665
Morrison JH, Magistretti PJ, Benoit R, Bloom FE (1984) The distribution and morphological characteristics of intracortical VIP-positive cell: an immunohistochemical analysis. Brain Res 292:269–282
Patton P, Thomas E, Wyatt R (1990) In: Winget K (ed) Computational dynamics of signal propagation in the visual cortex in Proceedings: Twenty-Sixth Semi-annual Cray User Group Meeting. Cray Users Group Bethpage, New York
Patton P, Thomas E, Wyatt R (1991) Computational model for space-time signal propagation in the visual cortex: II Dynamics of activity flow. Biol Cybern (in preparation)
Pellionisz A, Llinàs R (1977) A computer model of cerebellar purkinje cells. Neuroscience 2:37–48
Pellionisz A, Llinàs R, Perkel DH (1977) A computer model of the cerebellar cortex of the frog. Neuroscience 2:19–35
Peters A, Proskaur CC (1980) Synaptic relationships between a multipolar stellate cell and a pyramidal neuron in rat visual cortex: a combined Golgi-electron microscope study. J Neurocytol 9:163–183
Peters A, Regidor J (1981) A reassessment of the forms of nonpyramidal neurons in area 17 of cat visual cortex. J Comp Neurol 203:685–716
Rodieck RW (1979) Visual pathways. Ann Rev Neursci Ann Rev, Palo Alto, Calif, pp 193–225
Seagraves MA, Rosenquist AC (1982) The afferent and efferent callosal connections of retinotopically defined areas in cat cortex. J Neurosci 2:1090–1007
Schein SJ, de Monasterio FM (1987) Mapping of retinal and geniculate neurons onto striate cortex of macaque. J Neurosci 7:996–1009
Sejnowski T, Koch C, Churchland PS (1988) Computational neuroscience. Science 141:1299–1306
Shepherd GM (1988) In: Gazzaniga MS (ed) A basic circuit of cortical organization in perspectives in memory research. MIT Press, Cambridge Mass, pp 93–134
Sherman SM (1982) In: Morrison AR, Strick PL (eds). Parallel pathways in the cat's geniculocortical system: W-, X-, and Y cells in changing concepts of the nervous system.
Somogyi P, Cowey A (1981) Combined Golgi and electron microscope study of the synapses formed by double bouquet cells in the visual cortex of cat and monkey. J Comp Neurol 195:547–566
Somogyi P, Kisvarday ZF, Martin KAC, Whitteridge D (1983) Synaptic connections of morphologically identified and physiologically characterized large basket cells in the striate cortex of the cat. Neuroscience 10:261–294
Stone J, Dreher B (1982) Parallel processing of information in the visual pathway. TINS 5:441–446
Symonds LL, Rosenquist AC (1984) Corticocortical connections among visual areas in the cat. J Comp Neurol 229:1–38
Symonds LL, Rosenquist AC, Edwards SB and Palmer LA (1981) Projections of the lateral posterior complex to visual cortical areas in the cat. Neuroscience 6:1195–2020
Szentagothai J (1978) Specificity versus (quasi-) randomness in cortical connectivity. In: Brazier MAB, Petsche H (eds) Architectonics of cerebral cortical connectivity. Raven Press, New York, pp 77–97
Tank DW (1990) In: Selverson AI (ed). Computations performed with oscillatory dynamics in vertebrate and invertebrate olfactory systems in neural computation. Society for Neuroscience, Washington D.C.
Thomson AM, Girdlestone D, David CW (1988) Voltage-dependent currents prolong single-axon post-synaptic potentials in layer III pyramidal neurons in rat neocortical slices. J Neurophysiol 60:1896–1907
Traub RD, Knowles WD, Miles R, Wong RKS (1987) Models of the cellular mechanism underlying preparation of epileptiform activity in the CA2-CA3 region of the hippocampal slice. Neuroscience 21:457–470
Traub RD, Miles R, Wong RKS (1988) Large scale simulations of the hippocampus. IEEE Eng Med Biol 7:31–51
Traub RD, Miles R, Wong RKS (1989) Model of the origin of rhymthmic population oscillations in the hippocampal slice. Science 243:1319–1325
Van der Loos H, Glaser H, Glaser EM (1972) Autapses in neocortex cerebri: synapses between a pyramidal cell's axon and its own dendrites. Brain Res 48:355–360
Wilson MA, Bower JM (1988) A computer simulation of olfactory cortex with functional implications for storage and retrieval of olfactory information. In: Anderson DZ (ed) Neural information processing systems. Americal Institute of Physics, New York
Wilson M, Bower JM (1989) In: Koch C, Segev I (eds) The simulation of large-scale neural networks in methods in neuronal modeling. MIT Press, Cambridge Mass, pp 291–333
Winfield DA, Gatter KC, Powell TPS (1980) An electron microscopic study of the types and proportions of neurons in the cortex of the motor and visual areas of the cat and rat. Brain 103:245–258
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from Cray Research Inc.
Rights and permissions
About this article
Cite this article
Thomas, E., Patton, P. & Wyatt, R.E. A computational model of the vertical anatomical organization of primary visual cortex. Biol. Cybern. 65, 189–202 (1991). https://doi.org/10.1007/BF00198090
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00198090