Summary
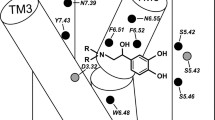
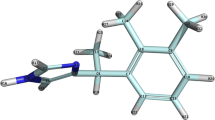
In recent biochemical studies it was demonstrated that residue Asp113 of theβ-adrenoceptor (β-AR) is an indispensable amino acid for the binding ofβ-AR antagonists. Earlier fluorescence studies showed that a tryptophan-rich region of theβ-AR is involved in the binding of propranolol, the prototypeβ-AR antagonist. Bearing these two biochemical findings in mind, we explored theβ-AR part containing Asp113, for an energetically favorable antagonist binding site. This was done by performing molecular docking studies with the antagonist propranolol and a specificβ-AR peptide which included, besides Asp113, two possibly relevant tryptophan residues. In the docking calculations, the propranolol molecule was allowed to vary all its internal torsional angles. The receptor peptide was kept in anα-helix conformation, while side chains relevant to ligand binding were flexible to enable optimal adaptations to the ligand's binding conformation. By means of force-field calculations the total energy was minimized, consisting of the intramolecular energies of both ligand and receptor peptide, and the intermolecular energy. We found an antagonist binding site, consisting of amino acids Asp113 and Trp109, which enabled energetically favorable interactions with the receptor-binding groups of propranolol. According to these results, binding involves three main interaction points: (i) a reinforced ionic bond; (ii) a hydrogen bond; and (iii) a hydrophobic/charge transfer interaction. The deduced binding site shows a difference in affinity between the levo- and dextrorotatory isomers of propranolol caused by a difference in ability to form a hydrogen bond, which is in conformity with the experimentally observed stereoselectivity. Moreover, it also provides an explanation for theβ 1-selectivity ofp-phenyl substituted phenoxypropanolamines like betaxolol. Thep-phenyl substituent of betaxolol was shown to be sterically hindered upon binding to theβ 2-AR peptide, whereas this hindrance is very likely to be much less with theβ 1-AR peptide. Finally, the proposed antagonist binding site is discussed in the light of some recent biochemical findings and theories.
Similar content being viewed by others
Abbreviations
- β-AR:
-
β-adrenergic receptor
- cDNA:
-
complementary DNA
- H-bond:
-
hydrogen bond
- VdW:
-
van der Waals
- QSAR:
-
quantitative structure-activity relationship
- 125I-pBABC:
-
p-(bromoacetamido)benzyl-1-[125I]iodocarazol
References
Dohlman, H.G., Caron, M.G. and Lefkowitz, R.J., Biochemistry, 26 (1987) 2657–2664.
Stiles, G.L., Caron, M.G. and Lefkowitz, R.J., Physiol. Rev., 64 (1984) 661–742.
Dixon, R.A.F., Kobilka, B.K., Strader, D.J., Benovic, J.L., Dohlman, H.G., Frielle, T., Bolanowski, M.A., Bennett, C.D., Rands, E., Diehl, R.E., Mumford, R.A., Slater, E.E., Sigal, I.S., Caron, M.G., Lefkowitz, R.J. and Strader, C.D., Nature, 321 (1986) 75–79.
Kobilka, B.K., Dixon, R.A.F., Frielle, T., Dohlman, H.G., Bolanowski, M.A., Sigal, I.S., Yang-Feng, T.L., Francke, U., Caron, M.G. and Lefkowitz, R.J., Proc. Nat. Acad. Sci. U.S.A., 84 (1987) 46–50.
Yarden, Y., Rodriguez, H., Wong, S.K.-F., Brandt, D.R., May, D.C., Burnier, J., Harkins, R.N., Chen, E.Y., Ramachandran, J., Ullrich, A. and Ross, E.M., Proc. Nat. Acad. Sci. U.S.A., 83 (1986) 6795–6799.
Frielle, T., Collins, S., Daniel, K.W., Caron, M.G., Lefkowitz, R.J. and Kobilka, B.K., Proc. Nat. Acad. Sci. U.S.A., 84 (1987) 7920–7924.
Dohlman, H.G., Bouvier, M., Benovic, J.L., Caron, M.G. and Lefkowitz, R.J., J. Biol. Chem., 262 (1987) 14282–14288.
Dixon, R.A.F., Sigal, I.S., Rands, E., Register, R.B., Candelore, M.R., Blake, A.D. and Strader, C.D., Nature, 326 (1987) 73–77.
Strader, C.D., Sigal, I.S., Register, R.B., Candelore, M.R., Rands, E. and Dixon, R.A.F., Proc. Nat. Acad. Sci., U.S.A., 84 (1987) 4384–4388.
Strader, C.D., Sigal, I.S., Candelore, M.R., Rands, E., Hill, W.S. and Dixon, R.A.F., J. Biol. Chem., 263 (1988) 10267–10271.
Ijzerman, A.P., Aué, G.H.J., Bultsma, T., Linschoten, M.R. and Timmerman, H., J. Med. Chem., 28 (1985) 1328–1334.
Cherksey, B.D., Murphy, R.B. and Zadunaisky, J.A., Biochemistry, 20 (1981) 4278–4283.
Ijzerman, A.P. and Van Vlijmen, H.W.Th., J. Comput.-Aided Mol. Design, 2 (1988) 43–53.
Chem-X: Molecular modeling system, Chemical Design Ltd., Oxford, U.K.
CSD: Cambridge Crystallopgraphic Database, Cambridge Crystallographic Data Center, Lensfield Road, Cambridge, U.K.
Stewart, J.P., MOPAC: A general molecular orbital package, QCPE Bull., 3 (1983) 43.
Allinger, N.L. and Yuh, Y.H., QCPE Program No. 395, MM2.
Gasteiger, J. and Marsili, M., Tetrahedron, 36 (1980) 3219–3228.
Morris, T.H. and Kaumann, A.J., Naunyn-Schmied. Arch. Pharmacol., 327 (1984) 176–179.
Andrews, P.R., Craik, D.J. and Martin, J.L., J. Med. Chem., 27 (1984) 1648–1657.
Ijzerman, A.P., Dorlas, R., Aué, G.H.J., Bultsma, T. and Timmerman, H., Biochem. Pharmacol., 34 (1985) 2883–2890.
Dohlman, H.G., Caron, M.G., Strader, C.D., Amlaiky, N. and Lefkowitz, R.J., Biochemistry, 27 (1988) 1813–1817.
Hargrave, P.A., McDowell, J.H., Feldmann, R.J., Atkinson, P.H., Rao, J.K.M. and Argos, P., Vision Res., 24 (1984) 1487–1499.
Applebury, M.L. and Hargrave, P.A., Vision Res., 26 (1986) 1881–1895.
Wheatley, M., Hulme E.C., Birdsall, N.J.M., Curtis, C.A.M., Eveleigh, P., Pedder, E.K. and Poyner, D., In Levine R.R., Birdsall, N.J.M., North, R.A., Holman, M., Watanabe, A. and Iversen, L.L. (Eds.) Subtypes of Muscarinic Receptors III, Proc. Third Int. Symp. Subtypes of Muscarinic Receptors, Elsevier Publications, Cambridge, 1988, pp. 19–24.
Albert, A., Selective Toxicity and Related Topics 4th ed., Methuen & Co. Ltd., London, 1971, pp. 182–187.
Kobilka, B.K., Matsui, H., Kobilka, T.S., Yang-Feng, T.L., Francke, U., Caron, M.G., Lefkowitz, R.J. and Regan, J.W., Science, 238 (1987) 650–656.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van Vlijmen, H.W.T., Ijzerman, A.P. Molecular modeling of a putative antagonist binding site on helix III of the β-adrenoceptor. J Computer-Aided Mol Des 3, 165–174 (1989). https://doi.org/10.1007/BF01557726
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01557726