Abstract
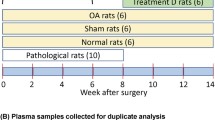
Osteoarthritis (OA) is a prevalent disorder among the elderly, which is characterized by the degradation of articular cartilage, leading to joint erosion and pain. The release of hydroxyproline, a major component of collagen, into body fluids is a marker of cartilage degradation. Still, an increasing number of people are studying bioanalytical methods and validation for the estimation of hydroxyproline. Yet, there are still no consistent conclusions about OA patients. Blood contains a massive number of metabolites that can potentially become biomarkers, but the metabolome coverage of current analytical techniques remains insufficient. Clear knowledge related to this important issue would definitely facilitate a better understanding and validation of the hydroxyproline. Therefore, this study aims to develop and validate a reliable LC–MS/MS method for estimating hydroxyproline levels in urine samples of osteoarthritic patients. The method demonstrated high sensitivity, selectivity, and precision, with a linear range of 10 ng–320 ng ml−1 and a correlation coefficient (r2) of 0.9993. Sample preparation involved hydrolyzing urine samples and optimizing chromatographic conditions for effective separation. The method validation followed ICH M10 guidelines, ensuring accuracy, precision, and stability. The results indicated that hydroxyproline is a significant biomarker for assessing cartilage degradation severity, thus aiding in the effective management of OA. The developed LC–MS/MS method provides a tool for diagnosing and monitoring OA, thus providing sensitivity to disease progression and treatment efficacy. This method's high-throughput capability is essential for large-scale clinical studies, enhancing the understanding of OA and improving patient outcomes.






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the manuscript.
Code availability
Not applicable.
Abbreviations
- LC–MS/MS:
-
Liquid chromatography–mass spectroscopy/mass spectroscopy
- ACN:
-
Acetonitrile
- CN:
-
Cyano
- RSD:
-
Relative standard deviation
References
Biondi PA, Chiesa LM, Storelli MR, Renon P. A new procedure for the specific high-performance liquid chromatographic determination of hydroxyproline. J Chromatogr Sci. 1997;35(11):509–12. https://doi.org/10.1093/chromsci/35.11.509.
Jones BQ, Covey CJ, Sineath MH Jr. Nonsurgical management of knee pain in adults. Am Fam Physician. 2015;92(10):875–83 (PMID: 26554281).
Kindt E, Gueneva-Boucheva K, Rekhter MD, Humphries J, Hallak H. Determination of hydroxyproline in plasma and tissue using electrospray mass spectrometry. J Pharm Biomed Anal. 2003;33(5):1081–92. https://doi.org/10.1016/s0731-7085(03)00359-5.
Kivirikko KI. Urinary excretion of hydroxyproline in health and disease. Int Rev Connect Tissue Res. 1970;5:93–163. https://doi.org/10.1016/b978-0-12-363705-5.50008-7.
Langrock T, García-Villar N, Hoffmann R. Analysis of hydroxyproline isomers and hydroxylysine by reversed-phase HPLC and mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2007;847(2):282–8. https://doi.org/10.1016/j.jchromb.2006.10.015.
Legido-Quigley C, Stella C, Perez-Jimenez F, Lopez-Miranda J, Ordovas J, Powell J, van-der-Ouderaa F, Ware L, Lindon JC, Nicholson JK, Holmes E. Liquid chromatography-mass spectrometry methods for urinary biomarker detection in metabonomic studies with application to nutritional studies. Biomed Chromatogr. 2010;24(7):737–43. https://doi.org/10.1002/bmc.1357.
Manek NJ, Lane NE. Osteoarthritis: current concepts in diagnosis and management. Am Fam Physician. 2000;61(6):1795–804.
McLafferty FW. Mass spectrometric analysis. Molecular rearrangements. Anal Chem. 1959;31:82–7.
Mei H, Hsieh Y, Nardo C, Xu X, Wang S, Ng K, Korfmacher WA. Investigation of matrix effects in bioanalytical high-performance liquid chromatography/tandem mass spectrometric assays: application to drug discovery. Rapid Commun Mass Spectrom. 2003;17(1):97–103. https://doi.org/10.1002/rcm.876.
Reed P, Holbrook IB, Gardner ML, McMurray JR. Simple, optimized liquid-chromatographic method for measuring total hydroxyproline in urine evaluated. Clin Chem. 1991;37(2):285–90.
Rydziel S, Canalis E. Analysis of hydroxyproline by high performance liquid chromatography and its application to collagen turnover studies in bone cultures. Calcif Tissue Int. 1989;44(6):421–4. https://doi.org/10.1007/BF02555971.
Smith R. Collagen and disorders of bone. Clin Sci (London, England: 1979). 1980;59(4):215–23. https://doi.org/10.1042/cs0590215.
Tiwari G, Tiwari R. Bioanalytical method validation: an updated review. Pharm Methods. 2010;1(1):25–38. https://doi.org/10.4103/2229-4708.72226.
Zhou S, Song Q, Tang Y, Naidong W. Critical review of development, validation, and transfer for high throughput bioanalytical LC-MS/MS methods. Curr Pharm Anal. 2005;1:3–14.
Funding
No funds, grants were received by any of the authors.
Author information
Authors and Affiliations
Contributions
All author is contributed to the design and methodology of this study, the assessment of the outcomes and the writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tallam, A.K., Reddy, K.T.K., Panigrahy, U.P. et al. Bioanalytical Method Development and Validation for the Estimation of Hydroxyproline in Urine Samples of Osteoarthritic Patients Using LC–MS/MS Technique. SN COMPUT. SCI. 5, 884 (2024). https://doi.org/10.1007/s42979-024-03237-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42979-024-03237-x