Abstract
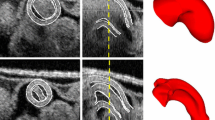
Heart morphogenesis and growth are influenced by hemodynamic forces (wall shear stress and blood pressure) acting on the walls of the heart. Mechanisms by which hemodynamic forces affect heart development are not well understood, in part because of difficulties involved in measuring these forces in vivo. In this paper, we show how wall shear stress in the heart outflow tract (OFT) of chick embryos at an early developmental stage (HH18) are affected by changes in the geometry and motion of the OFT wall. In particular, we were interested in the effects of cardiac cushions, which are protrusions of the OFT wall toward the lumen and that are located where valves will later form. We developed idealized finite element models (FEM) of the chick OFT with and without cardiac cushions. Geometrical parameters used in these models were estimated from in vivo images obtained using optical coherence tomography (OCT) techniques. The FEMs showed significant reverse blood flow (backflow) in the OFT, consistent with experimental observations in the chick heart at HH18, and revealed that cardiac cushions decrease backflow. In addition, our FEMs showed that the spatial distribution of wall shear stress is affected by cardiac cushions, with larger absolute peak values observed at the cushions. Differences in mechanical stimuli (wall shear stress) that the cells in the cardiac cushions and elsewhere are subjected to may affect valve formation and heart development.










Similar content being viewed by others
References
Bartman T, Hove J (2005) Mechanics and function in heart morphogenesis. Dev Dyn 233:373–381
Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (1997) Unilateral vitelline vein ligation alters intracardiac blood flow patterns and morphogenesis in the chick embryo. Circ Res 80:473–481
Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE (1999) Extraembryonic venous obstructions lead to cardiovascular malformations and can be embryolethal. Cardiovasc Res 41:87–99
Hove JR, Köster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421:172–177
Lee JS, Yu Q, Shin JT, Sebzda E, Bertozzi C, Chen M, Mericko P, Stadtfeld M, Zhou D, Cheng L, Graf T, MacRae CA, Lepore JJ, Lo CW, Kahn ML (2006) Klf2 is an essential regulator of vascular hemodynamic forces in vivo. Dev Cell 11:845–857
Hoffman JIE (1995) Incidence of congenital heart disease. II. Prenatal incidence. Pediatr Cardiol 16:155–165
Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S, Lloyd-Jones D, Goff DJ Jr, Hong Y, Adams R, Friday G, Furie K, Gorelick P, Kissela B, Marler J, Meigs J, Roger V, Sidney S, Sorlie P, Steinberger J, Wasserthiel-Smoller S, Wilson M, Wolf P (2006) Heart disease and stroke statistics-2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circ 113:85–151
Fisher AB, Chien S, Barakat AI, Nerem RM (2001) Endothelial cellular response to altered shear stress. Am J of Physiol Lung Cell Mol Physiol 281:529–533
Davies PF (1995) Flow-mediated endothelial mechanotransduction. Physiol Rev 75:519–560
Li YS, Haga JH, Chien S (2005) Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech 38:1949–1971
Miller CE, Donlon KJ, Toia L, Wong CL, Chess PR (2000) Cyclic strain induces proliferation of cultured embryonic heart cells. In Vitro Cell Dev Biol-Animal 36:633–639
Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ (1989) Effect of increased pressure on ventricular growth in stage 21 chick embryos. Am J Physiol 257 (Heart Circ. Physiol. 26): 55–61
Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB (1999) Remodeling of chick embryonic ventricular myoarchitecture under experimentally changed loading conditions. Anat Rec 254:238–252
Groenendijk BC, Hierck BP, Vrolijk J, Baiker M, Pourquie MJ, Gittenberger-de Groot AC, Poelmann RE (2005) Changes in shear stress-related gene expression after experimentally altered venous return in the chicken embryo. Circ Res 96:1291–1298
Lucitti JL, Tobita K, Keller BB (2005) Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. J Exp Biol 208:1877–1885
Hu N, Keller BB (1995) Relationship of simultaneous atrial and ventricular pressures in stage 16–27 chick embryos. Heart Circ Physiol 38:1359–1362
Vennemann P, Kiger KT, Lindken R, Groenendijk BC, Stekelenburg-de Vos S, ten Hagen TL, Ursem NT, Poelmann RE, Westerweel J, Hierck BP (2006) In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart. J Biomech 39:1191–1200
Hove JR (2006) Quantifying cardiovascular flow dynamics during early development. Pediatr Res 60:6–13
DeGroff CG, Thornburg BL, Pentecost JO, Thornburg KL, Gharib M, Sahn DJ, Baptista A (2003) Flow in the early embryonic human heart: a numerical study. Pediatr Cardiol 24:375–380
Loots E, Hillen B, Veldman AEP (2003) The role of hemodynamics in the development of the outflow tract of the heart. J Eng Math 45:91–104
Hamburger V, Hamilton HL (1951) A series of normal stages in the development of the chick embryo. J Morphol 88(1):49–92
Clark EB, Hu N (1982) Developmental hemodynamic changes in the chick embryo from stage 18 to 27. Circ Res 51:810–815
Martinsen BJ (2005) Reference guide to the stages of chick heart embryology. Dev Dyn 233:1217–1237
Rothenberg F, Fisher SA, Watanabe M (2003) Sculpting the cardiac outflow tract. Birth Defects Res C Embryo Today 69:38–45
Clark EB (1984) Functional aspects of cardiac development. In: Zak R (ed) Growth of the Heart in Health and Disease. Raven Press, New York, pp 81–103
de Jong F, Opthof T, Wilde AA, Janse MJ, Charles R, Lamers WH, Moorman AF (1992) Persisting zones of slow impulse conduction in developing chicken hearts. Circ Res 71:240–250
Männer J (2000) Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259:248–262
Qayyum SR, Webb S, Anderson RH, Verbeek FJ, Brown NA, Richardson MK (2001) Septation and valve formation in the outflow tract of the embryonic chick heart. Anat Rec 264:273–283
Keller BB, Hu N, Clark EB (1990) Correlation of ventricular area, perimeter and conotruncal diameter with ventricular mass and function in the chick embryo from stages 12 to 24. Circ Res 66:109–114
Hu N, Clark EB (1989) Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65:1665–1670
Davies PF, Zilberberg J, Helmke BP (2003) Spatial microstimuli in endothelial mechanosignaling. Circ Res 92:359–370
White CR, Haidekker M, Bao X, Frangos JA (2001) Temporal gradients in shear, but not spatial gradients stimulate endothelial cell proliferation. Circ 103:2508–2513
Liu A, Rugonyi S, Pentecost JO, Thornburg KL (2007) Finite element modeling of blood flow-induced mechanical forces in the outflow tract of chick embryonic hearts. Comput Struct 85:727–738
Wang RK, Ma ZH (2006) A practical approach to eliminate autocorrelation artifacts for volume-rate spectral domain optical coherence tomography. Phys Med Biol 51:3231–3239
Wang RK (2007) In vivo full range complex Fourier Domain optical coherence tomography. Appl Phys Lett 90:1–3
Wang RK, Ma ZH (2006) Real time flow imaging by removing texture pattern artifacts in spectral domain optical Doppler tomography. Opt Lett 31:3001–3003
Donea J (1983) Arbitrary Lagrangian–Eulerian finite element methods. In: Belytschko T, Hughes T (eds) Computational methods for transient analysis. Elsevier, Amsterdam, pp 474–515
Rugonyi S, Bathe KJ (2001) On finite element analysis of fluids flows fully coupled with structural interactions. CMES 2:195–212
Chadwick P (1976) Continuum Mechanics: Concise Theory and Problems. Dover Publications Inc, New York
Hogers B, DeRuiter MC, Baasten AM, Gittenberger-de Groot AC, Poelmann RE (1995) Intracardiac blood flow patterns related to the yolk sac circulation of the chick embryo. Circ Res 76:871–877
ADINA R&D (2006) Adina System Online Manuals. Adina R&D Inc, Massachusetts, USA
Bathe KJ, Zhang H (2002) A flow-condition-based interpolation finite element procedure for incompressible fluid flows. Comput Struct 80:1267–1277
White FM (2006) Viscous Fluid Flow. McGraw-Hill, New York
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation: positive correlation between the plaque location and low oscillating shear stress. Arterioscl 5:293–302
Acknowledgments
This work was partially supported by a Beginning Grant-in-Aid from the Pacific Mountain Affiliate of the American Heart Association (0760063Z).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, A., Wang, R.K., Thornburg, K.L. et al. Dynamic variation of hemodynamic shear stress on the walls of developing chick hearts: computational models of the heart outflow tract. Engineering with Computers 25, 73–86 (2009). https://doi.org/10.1007/s00366-008-0107-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00366-008-0107-0