Abstract
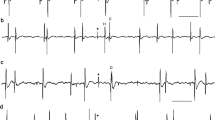
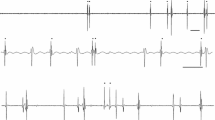
Recurrent inhibition between tonically activated single human motoneurons was studied experimentally and by means of a computer simulation. Motor unit activity was recorded during weak isometric constant-force muscle contractions of brachial biceps (BB) and soleus (SOL) muscles. Three techniques (cross correlogram, frequencygram, and interspike interval analysis) were used to gauge the relations between single motor unit potential trains. Pure inhibition was detected in 5.6% of 54 BB motoneuron pairs and in 5.2% of 43 SOL motoneuron pairs. In 27.8% (BB) and 23.7% (SOL) presumed inhibition symptoms were accompanied by a synchrony peak; 37% (BB) and 48.8% (SOL) exhibited synchrony alone. The demonstrated inhibition was very weak, at the edge of detectability. Computer simulations were based on the threshold-crossing model of a tonically firing motoneuron. The model included synaptic noise as well as threshold and postsynaptic potential (PSP) amplitude change within interspike interval. Inhibition efficiency of the model neurons increased with IPSP amplitude and duration, and with increasing source firing rate. The efficiency depended on target motoneuron interspike interval in a manner similar to standard deviation of ISI. The minimum detectable amplitude estimated in the simulations was about 50μV, which, compared with the experimental results, suggests that amplitudes of detectable recurrent IPSPs in human motoneurons during weak muscle contractions do not exceed this magnitude. Since recurrent inhibition is known to be progressively depressed with an increase in the force of voluntary contraction, it is concluded that the recurrent inhibition hardly plays any important role in the isometric muscle contractions of constant force.
Similar content being viewed by others
References
Ashby P, Zilm D (1982) Relationship between EPSP shape and cross-correlation profile explored by computer simulation for studies on human motoneurons. Exp Brain Res 47:33–40
Baker JR, Davey NJ, Ellaway PH, Friedland CL (1992) Short-term synchrony of motor unit discharge during weak isometric contraction in Parkinson’s disease. Brain 115:137–154
Baldissera F, Gustafsson B (1974) Afterhyperpolarization conductance time course in lumbar motoneurones of the cat. Acta Physiol Scand 91:512–527
Bremner FD, Baker JR, Stephens JA (1991) Effect of task on the degree of synchronization of intrinsic hand muscle motor units in man. J Neurophysiol 66:2072–2083
Bussel B, Pierrot-Deseilligny E (1977) Inhibition of human motoneurons, probably of Renshaw origin, elicited by an orthodromic motor discharge. J Physiol Lond 269:319–339
Calvin WH (1974) Three modes of repetitive firing and the role of threshold time course between spikes. Brain Res 69:341–346
Calvin WH, Stevens CF (1968) Synaptic noise and other sources of randomness in motoneuron interspike intervals. J Neurophysiol 31:574–587
Calvin WH, Schwindt PC (1972) Steps in production of motoneuron spikes during rhythmic firing. J Neurophysiol 35:297–310
Cullheim S, Kellerth J-O (1978) A morphological study of the axons and recurrent collaterals of cat α-motoneurones as differentiated morphologically. J Physiol Lond 281:301–313
Datta AK, Stephens JA (1979) The effect of digital nerve stimulation on motor unit interspike intervals recorded during voluntary contraction of first dorsal interosseous muscle in man [proceedings]. J Physiol 292:16P–17P
Datta AK, Stephens JA (1990) Synchronization of motor unit activity during voluntary contraction in man. J Physiol 422:397–419
Datta AK, Farmer SF, Stephens JA (1991) Central nervous pathways underlying synchronization of human motor unit firing studied during voluntary contractions. J Physiol 432:401–425
Davey NJ, Ellaway PH, Baker JR, Friedland CL (1993) Rhythmicity associated with a high degree of short-term synchrony of motor unit discharge in man. Exp Physiol 78:649–661
Davey NJ, Ellaway PH, Friedland CL, Short DJ (1990) Motor unit discharge characteristics and short term synchrony in paraplegic humans. J Neurol Neurosurg Psychiatry 53:764–769
Eccles JC (1966) The ionic mechanisms of excitatory and inhibitory synaptic action. Ann NY Acad Sci 137:473–494
Ellaway PH (1978) Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol 45:302–304
Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR (1997) A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods 74:175–187
Fetz EE, Gustafsson B (1983) Relation between shapes of post-synaptic potentials and changes in firing probability of cat motoneurones. J Physiol 341:387–410
Hamm TM, Sasaki S, Stuart DG, Windhorst U, Yuan CS (1987) The measurement of single motor-axon recurrent inhibitory post-synaptic potentials in the cat. J Physiol 388:631–651
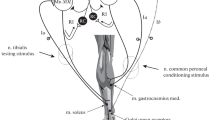
Hultborn H, Pierrot-Deseilligny E (1979) Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. J Physiol 297:229–251
Hultborn H, Pierrot-Deseilligny E, Wigstrom H (1979) Recurrent inhibition and afterhyperpolarization following motoneuronal discharge in the cat. J Physiol 297:253–266
Katz R, Pierrot-Deseilligny E (1999) Recurrent inhibition in humans. Prog Neurobiol 57:325–355
Kernell D (1984) The meaning of discharge rate: excitation-to-frequency transduction as studied in spinal motoneurones. Arch Ital Biol 122:5–15
Kernell D, Bakels R, Copray JC (1999) Discharge properties of motoneurones: how are they matched to the properties and use of their muscle units? J Physiol Paris 93:87–96
Kudina LP (1980) Reflex effects of muscle afferents on antagonist studied on single firing motor units in man. Electroencephalogr Clin Neurophysiol 50:214–221
Kudina LP (1988) Excitability of firing motoneurones tested by Ia afferent volleys in human triceps surae. Electroencephalogr Clin Neurophysiol 69:576–580
Kudina LP, Pantseva RE (1988) Recurrent inhibition of firing motoneurones in man. Electroencephalogr Clin Neurophy- siol 69:179–185
Lindsay AD, Binder MD (1991) Distribution of effective synaptic currents underlying recurrent inhibition in cat triceps surae motoneurons. J Neurophysiol 65:168–177
Maltenfort MG, Heckman CJ, Rymer WZ (1998) Decorrelating actions of Renshaw interneurons on the firing of spinal motoneurons within a motor nucleus: a simulation study. J Neurophysiol 80:309–323
Mattei B, Schmied A, Mazzocchio R, Decchi B, Rossi A, Vedel JP (2003) Pharmacologically induced enhancement of recurrent inhibition in humans: effects on motoneurone discharge patterns. J Physiol 548:615–629
Matthews PB (1999a) The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. J Physiol 518:867–882
Matthews PB (1999b) Properties of human motoneurones and their synaptic noise deduced from motor unit recordings with the aid of computer modelling. J Physiol Paris 93:135–145
Mazurkiewicz L, Piotrkiewicz M (2004) Investigation of healthy and sick motoneuron properties by means of a system for identification and analysis of motor unit potential trains. Biocybern Biomed Eng (in press)
Meunier S, Pierrot-Deseilligny E, Simonetta-Moreau M (1994) Pattern of heteronymous recurrent inhibition in the human lower limb. Exp Brain Res 102:149–159
Miles TS, Le TH, Turker KS (1989) Biphasic inhibitory responses and their IPSPs evoked by tibial nerve stimulation in human soleus motor neurones. Exp Brain Res 77:637–645
Moore GP, Perkel DH, Segundo JP (1966) Statistical analysis and functional interpretation of neuronal spike data. Annu Rev Physiol 28:493–522
Nelson PG (1966) Interaction between spinal motoneurons of the cat. J Neurophysiol 29:275–287
Nordstrom MA, Fuglevand AJ, Enoka RM (1992) Estimating the strength of common input to human motoneurons from the cross-correlogram. J Physiol 453:547–574
Pantseva RE (1984) Study of recurrent inhibition during orthodromic stimulation of motoneurons. Hum Physiol 10:184–188
Person RS, Kozhina GV (1978) Study of orthodromic and antidromic effects of nerve stimulation on single motoneurones of human hand muscles. Electromyogr Clin Neurophysiol 18:437–456
Pierrot-Deseilligny E, Bussel B (1975) Evidence for recurrent inhibition by motoneurons in human subjects. Brain Res 88:105–108
Piotrkiewicz M (1999) An influence of afterhyperpolarization on the pattern of motoneuronal rhythmic activity. J Physiol Paris 93:125–133
Piotrkiewicz M, Churikova L, Person R (1992) Excitability of single firing human motoneurones to single and repetitive stimulation (experiment and model). Biol Cybern 66:253–259
Piotrkiewicz M, Kudina L, Hausmanowa-Petrusewicz I, Zhoukovskaya N, Mierzejewska J (2001) Discharge properties and afterhyperpolarization of human motoneurons. Biocybern Biomed Eng 21:53–75
Powers RK, Binder MD (1996) Experimental evaluation of input-output models of motoneuron discharge. J Neurophysiol 75:367–379
Powers RK, Binder MD (2000) Relationship between the time course of the afterhyperpolarization and discharge variability in cat spinal motoneurones. J Physiol 528:131–150
Renshaw B (1941) Influence of discharge of motoneurons upon excitation of neighboring motoneurones. J Neurophysiol 4:167–183
Reyes AD, Fetz EE (1993) Two modes of interspike interval shortening by brief transient depolarizations in cat neocortical neurons. J Neurophysiol 69:1661–1672
Schmied A, Pagni S, Sturm H, Vedel JP (2000) Selective enhancement of motoneurone short-term synchrony during an attention-demanding task. Exp Brain Res 133:377–390
Stuart G, Sakmann B (1995) Amplification of EPSPs by axosomatic sodium channels in neocortical pyramidal neurons. Neuron 15:1065–1076
Turker KS, Powers RK (1999) Effects of large excitatory and inhibitory inputs on motoneuron discharge rate and probability. J Neurophysiol 82:829–840
Turker KS, Schmied A, Cheng HB (1996) Correlated changes in the firing rate of human motor units during voluntary contraction. Exp Brain Res 111:455–464
Uchiyama T, Johansson H, Windhorst U (2003a) A model of the feline medial gastrocnemius motoneuron-muscle system subjected to recurrent inhibition. Biol Cybern 89:139–151
Uchiyama T, Johansson H, Windhorst U (2003b) Static and dynamic input-output relations of the feline medial gastrocnemius motoneuron-muscle system subjected to recurrent inhibition:a model study. Biol Cybern 89:264–273
Veale JL, Rees S (1973) Renshaw cell activity in man. J Neurol Neurosurg Psychiatry 36:674–683
Westgaard RH, De Luca CJ (2001) Motor control of low-threshold motor units in the human trapezius muscle. J Neurophysiol 85:1777–1781
Windhorst U (1990) Activation of Renshaw cells. Prog Neurobiol 35:135–179
Windhorst U (1996) On the role of recurrent inhibitory feedback in motor control. Prog Neurobiol 49:517–587
Zengel JE, Reid SA, Sypert GW, Munson JB (1983) Presynaptic inhibition, EPSP amplitude, and motor-unit type in triceps surae motoneurons in the cat. J Neurophysiol 49:922–931
Zengel JE, Reid SA, Sypert GW, Munson JB (1985) Membrane electrical properties and prediction of motor-unit type of medial gastrocnemius motoneurons in the cat. J Neurophysiol 53:1323–1344
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Piotrkiewicz, M., Kudina, L. & Mierzejewska, J. Recurrent inhibition of human firing motoneurons (experimental and modeling study). Biol. Cybern. 91, 243–257 (2004). https://doi.org/10.1007/s00422-004-0507-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-004-0507-1