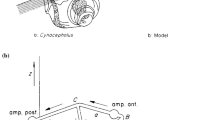
Abstract
In the present review, signal-processing capabilities of the canal lateral line organ imposed by its peripheral architecture are quantified in terms of a limited set of measurable physical parameters. It is demonstrated that cupulae in the lateral line canal organ can only partly be described as canal fluid velocity detectors. Deviation from velocity detection may result from resonance, and can be characterized by the extent to which a single dimensionless resonance number, N r , exceeds 1. This number depends on four physical parameters: it is proportional to cupular size, cupular sliding stiffness and canal fluid density, and inversely proportional to the square of fluid viscosity. Situated in a canal, a cupula may benefit from its resonance by compensating for the limited frequency range of water motion that is efficiently transferred into the lateral line canal. The peripheral transfer of hydrodynamic signals, via canal and cupula, leads to a nearly constant sensitivity to outside water acceleration in a bandwidth that ranges from d.c. to a cut-off frequency of up to several hundreds of Hertz, significantly exceeding the cut-off frequency of the lateral line canal. Threshold values of hydrodynamic detection by the canal lateral line organ are derived in terms of water displacement, water velocity, water acceleration and water pressure gradients and are shown to be close to the detection limits imposed by hair cell mechano-transduction in combination with the physical constraints of peripheral lateral line signal transfer. The notion that the combination of canal- and cupular hydrodynamics effectively provides the lateral line canal organ with a constant sensitivity to water acceleration at low frequencies so that it consequently functions as a low-pass detector of pressure gradients, supports the appropriateness of describing it as a sense organ that “feels at a distance” (Dijkgraaf in Biol Rev 38:51–105, 1963)
Similar content being viewed by others
References
Abdel-Latif H, Hassan ES, von Campenhausen C (1990) Sensory performance of blind Mexican cave fish after destruction of the canal neuromasts. Naturwissenschaften 77:237–239
Batchelor GK (1967) An introduction to fluid mechanics. Cambridge University Press, Cambridge
von Békésy G (1960) Experiments in hearing. ASA AIP report, McGraw-Hill, New York
Bleckmann H (1980) Reaction time and stimulus frequency in prey localization in the surface-feeding fish Aplocheilus lineatus. J Comp Physiol A 140:163–172
Bleckmann H (1993) Role of the lateral line in fish behaviour. In: Pitcher TJ (eds). Behaviour of Teleost fishes 2nd edn. Chapman & Hall, London, pp 201–246
Bleckmann H, Breithaupt T Blickhan R, Tautz J (1991) The time course and frequency content of hydrodynamic events caused by moving fish, frogs and crustaceans. J Comp Physiol A 168:749–757
de Boer E (1980) Auditory physics. Physical principles in hearing theory. I Phys reports 62:87–174
Cahn PH (1967) Lateral line detectors. Indiana University Press, Bloomington
Ćurčić-Blake B, van Netten SM (2005) Rapid responses of the cupula in the lateral line of ruffe (Gymnocephalus cernuus). J Comp Physiol A, 191:393–401
Coombs S, Görner P, Münz H (1989) The mechanosensory lateral line: neurobiology and evolution. Springer, Berlin Heidelberg New York
Coombs, S, Hastings, M, Finneran, JJ (1996) Modeling and measuring lateral line excitation patterns to changing dipole source locations. J Comp Physiol A 178:359–371
Coombs S, Janssen J (1990) Behavioral and neurophysiological assessment of lateral line sensitivity in the mottled sculpin, Cottus bairdi. J Comp Physiol A 167:557–567
Coombs S, Janssen J, Webb JF (1988) Diversity of lateral line systems: evolutionary and functional considerations. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds). Sensory biology of aquatic animals. Springer, Berlin Heidelberg New York, pp 553–593
Coombs S, Janssen J, Montgomery JC (1992) Functional and evolutionary implications of peripheral diversity in lateral line systems. In: Webster DB, Fay RR, Popper AN (eds) The evolutionary biology of hearing. Springer, Berlin Heidelberg New York, pp 267–294
Coombs S, Montgomery JC (1992) Fibers innervating different parts of the lateral line system of an Antarctic Notothenioid, Trematomus bernacchii, have similar frequency responses, despite large variations in the peripheral morphology. Brain Behav Evol 40:217–233
Coombs S, Montgomery JC (1999) The enigmatic lateral line system. In: Fay RR, Popper AN (eds) Comparative hearing: fish and amphibians. Springer, Berlin Heidelberg New York, pp 319–362
Corey DP, Garcia-Anoveros J, Holt JR, Kwan KY, Lin SY, Vollrath MA, Amalfitano A, Cheung EL, Derfler BH, Duggan A, Geleoc GS, Gray PA, Hoffman MP, Rehm HL, Tamasauskas D, Zhang DS (2004) TRPA1 is a candidate for the mechanosensitive transduction channel of vertebrate hair cells. Nature 432:723–730
Denton EJ, Blaxter, JHS (1976) The mechanical relationships between the clupeid swimbladder, inner ear and the lateral line. J Mar Biol Assoc UK 56:787–807
Denton EJ, Gray JAB (1982) The rigidity of fish and patterns of lateral line stimulation. Nature 297:679–681
Denton EJ, Gray JAB (1983) Mechanical factors in the excitation of clupeid lateral lines. Proc R Soc Lond B 218:1–26
Denton EJ, Gray JAB (1988) Mechanical factors in the excitation of the lateral line of fishes. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, Berlin Heidelberg New York, pp 595–617
Denton EJ, Gray JAB (1989) Some observations on the forces acting on neuromasts in fish lateral line canals. In: Coombs S, Görner P, Münz H (eds). The mechanosensory lateral line: neurobiology and evolution. Springer, Berlin Heidelberg New York, pp 229–246
Dijkgraaf S (1963) The functioning and significance of the lateral-line organs. Biol Rev 38:51–105
Elepfandt A (1982) Accuracy of taxis response to water waves in the clawed toad (Xenopus laevis Daudin) with intact or with lesioned lateral line system. J Comp Physiol 148:535–545
Engelmann J, Hanke W, Mogdans J, Bleckmann H (2000) Hydrodynamic stimuli and the fish lateral line. Nature 408:51–52
Engelmann J, Hanke W, Bleckmann H (2002) Lateral line reception in still- and running water. J Comp Physiol A 188:513–526
Enger PS, Kalmijn AJ, Sand O (1989) Behavioral investigations on the functions of the lateral line and inner ear in predation. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: neurobiology and evolution. Springer, Berlin Heidelberg New York, pp 575–587
Flock Å (1965) Electron microscopic and electro-physiological studies on the lateral line organ. Acta Oto-Laryngol Suppl 199:1–90
Géléoc GS, Lennan GW, Richardson GP, Kros CJ (1997) A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc R Soc Lond B 1997 264:611–621
Görner P (1963) Untersuchungen zur Morphologie und Electrophysiologie des Seitenlinienorgans vom Krallenfrosch (Xenopus laevis Daudin). Z Vergl Physiol 47:316–338
Harris GG, van Bergeijk WA (1962) Evidence that the lateral line organ responds to near field displacements of sound sources in water. J Acoust Soc Am 34:1831–1841
Harris GG, Frishkopf LS, Flock Å (1970) Receptor potentials from hair cells of the lateral line. Science 167:76–79
Hassan ES (1986) On the discrimination of spatial intervals by the blind cave fish (Anoptichthys jordani). J Comp Physiol A 159:701–710
Hoekstra D, Janssen J (1985) Non-visual feeding behavior of the mottled sculpin, Cottus bairdi, in Lake Michigan. Env Biol Fishes 12:111–117
Howard J, Hudspeth AJ (1988) Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1:189–199
Hudspeth AJ, ChoeY, Mehta AD, Martin P (2000) Putting ion channels to work: Mechanoelectrical transduction, adaptation, and amplification by hair cells. Proc Natl Acad Sci USA 97:11765–11772
Jielof R, Spoor A, de Vries H (1952) The microphonic activity of the lateral line. J Physiol 116:137–157
Kalmijn AJ (1988) Hydrodynamic and acoustic field detection. In: Atema J, Fay RR, Popper AN, Tavolga WN (eds) Sensory biology of aquatic animals. Springer, Berlin Heidelberg New York, pp 83–130
Kalmijn AJ (1989) Functional evolution of lateral line and inner ear sensory systems. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York, pp 187–215
Karlsen HE, Sand O (1987) Selective and reversible blocking of the lateral line in freshwater fish. J Exp Biol 133:249–263
Kelly JP, van Netten SM (1991) Topography and mechanics of the cupula in the fish lateral line. Variations of cupular structure and composition in three dimensions. J Morph 207:23–36
Kroese ABA, van der Zalm JM, van den Bercken J (1978) Frequency response of the lateral line organ of Xenopus laevis. Pfluegers Arch 375:167–175
Kroese ABA, van den Bercken J. (1980) Dual action of ototoxic antibiotics on sensory hair cells. Nature 283:395–397
Kroese ABA, van den Bercken J (1982) Effects of ototoxic antibiotics on sensory hair cell functioning. Hearing Res 6:183–97
Kroese ABA, van Netten SM (1989) Sensory transduction in lateral line sensory hair cells. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York, pp 265–284
Kroese ABA, Schellart NAM (1992) Velocity- and acceleration sensitive units in the trunk lateral line of the trout. J Neurophysiol 68:2212–2221
Kuiper JW (1956) The microphonic effect of the lateral line organ. PhD thesis, University of Groningen, The Netherlands
Lamb H (1932) Hydrodynamics. Reprint 6th edn. Dover, New- York
Landau LD, Lifshitz EM (1980) Statistical physics, Part 1 3rd edn. Pergamon Press, Oxford
Landau LD, Lifshitz EM (1987) Fluid mechanics 2nd edn. Pergamon Press, Oxford
Leydig F (1850) Ueber die Schleimkanäle der Knochenfische. Müll Arch Anat Physiol 170–181
Liff HJ, Shamres S (1972) Structure and motion of cupulae of lateral line organs in Necturus maculosus III. A technique for measuring the motion of free-standing lateral line cupulae.Q Progr Rep Res Lab Electr MIT 104:332–336
van Maarseveen JThPW (1994) Mechanophysiological investigation on the lateral line of the ruffe. PhD Thesis University of Groningen, The Netherlands
Markin VS, Hudspeth AJ (1995) Gating-spring models of mechanoelectrical transduction by hair cells of the internal ear. Annu Rev Biophys Biomol Struct 24:59–83
Martin P, Jülicher F, Hudspeth AJ (2003) The contribution of transduction channels and adaptation motors to the the hair cell’s active process. In: Gummer AW (eds) Biophysics of the cochlea: From molecules to models. World Scientific, Singapore, pp 3–15
Mathews J, Walker RL (1970) Mathematical methods of physics. Benjamin, New York
Montgomery JC, Baker CF, Carton AG (1997) The lateral line can mediate rheotaxis in fish. Nature 389:960–963
Montgomery JC, Coombs S, Janssen J (1994) Form and function relationships in lateral line systems: Comparative data from six species of Antarctic notothenioid fish. Brain Behav Evol 44:299–306
Montgomery JC, Macdonald JA (1987) Sensory tuning of lateral line receptors in Antartic fish to the movements of planktonic prey. Science 235:195–196
Münz H (1985) Single unit activity in the peripheral lateral line system of the cichlid fish Sarotherodon niloticus L. J Comp Physiol A 157:555–568
Münz H (1989) Functional organization of the lateral line periphery. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York New York, pp. 285–297
van Netten SM (1988) Laser interferometer microscope for the measurement of nanometer vibrational displacements of a light scattering microscopic object. J Acoust Soc Am 83:1667–1674
van Netten SM (1991) Hydrodynamics of the excitation of the cupula in the fish canal lateral line. J Acoust Soc Am 89:310–319
van Netten SM, Dinklo T, Marcotti W, Kros CJ (2003) Channel gating forces govern accuracy of mechano-electrical transduction in hair cells. Proc Natl Acad Sci USA 100:15510–15515
van Netten SM, Karlsson KJ, Khanna SM and Flock Å (1994) Effects of quinine on the mechanical frequency response of the cupula in the fish lateral line. Hearing Res 73:223–230
van Netten SM, Khanna SM (1994) Stiffness changes of the cupula associated with the mechanics of hair cells in the fish lateral line. Proc Natl Acad Sci USA 91:1549–1553
van Netten SM, Kroese ABA (1987) Laser interferometric measurements on the dynamic behavior of the cupula in the fish lateral line. Hearing Res 29: 55–61
van Netten SM, Kroese ABA (1989) Dynamic behavior and micromechanical properties of the cupula. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York New York, pp 247–263
van Netten SM., Kros CJ (2000). Gating energies and forces of the mammalian hair cell transducer channel and related hair bundle mechanics. Proc R Soc Lond B Biol Sci 267:1915–1923
van Netten SM, van Maarseveen JThPW (1994) Mechanophysiological properties of the supraorbital lateral line canal in ruffe (Acerina Cernua L.) Proc R Soc Lond B 256:239–246
van Netten SM (1997) Hair cell mechano-transduction: Its influence on the gross mechanical characteristics of a hair cell organ. Biophys Chem 68:43–52
Nicolson T, Rusch A, Friedrich RW, Granato M, Ruppersberg JP, Nusslein-Volhard C (1998) Genetic analysis of vertebrate sensory hair cell mechanosensation: the zebrafish circler mutants. Neuron 20:271–83
Northcutt RG (1989) The phylogenetic distribution and innervation of craniate mechanoreceptive lateral lines. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York New York, pp 17–78
Olson ES (1998) Observing middle and inner ear mechanics with novel intracochlear pressure sensors. J Acoust Soc Am 103:3445–3463
Partridge BL, Pitcher TJ (1980) The sensory basis of fish schools: relative roles of lateral line and vision. J Comp Phys 135:315–325
Ricci AJ, Crawford AC, Fettiplace R. (2002) Mechanisms of active hair bundle motion in auditory hair cells. J Neurosci. 22:44–52
Russell IJ (1976) Amphibian lateral line receptors. In: Llinas R, Precht W (eds) Frog Neurobiology. Springer, Berlin Heidelberg New York, pp 513–550
Russell IJ, Kossl M, Richardson GP (1992). Nonlinear mechanical responses of mouse cochlear hair bundles. Proc R Soc Lond B Biol Sci 250:217–27
Sand O (1984) Lateral-line systems. In: BolisL, Keynes RD, Maddrell SHP (eds) Comparative physiology of sensory systems. Cambridge University Press, London pp 3–32
Schlichting H (1979) Boundary layer theory 7th edn. McGraw-Hill, New York
Schulze FE (1861) Über die Nervenendigung in den sogenannten Schleimkanälen der Fische und über entsprechende Organe der durch Kiemen athmenden Amphibien. Arch Anat Physiol Lpz 759–769
Sexl T (1930) Über den von E.G. Richardson entdeckten “Annulareffekt”. Z Phys 61:349–362
Sidi S, Friedrich RW, Nicolson T (2003). NompC TRP channel required for vertebrate sensory hair cell mechanotransduction. Science 301:96–99
Söllner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, The Tübingen (2000) Screen Consortium, Müller U, Nicolson T (2004) Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428:955–959
Stokes GG (1851) On the effect of the internal friction of fluids on the motion of pendulums. Trans Camb Phil Soc 9:6–106
Strelioff D, Honrubia V (1978) Neural transduction in Xenopus Laevis lateral line system. J Neurophysiol 41:432–444
Tsang PTSK (1997) Laser interferometric flow measurements in the lateral line organ. PhD thesis, University of Groningen, The Netherlands
Tsang PTSK, van Netten SM (1997) Fluid flow profiles measured in the supraorbital lateral line canal of the ruff. In: Lewis ER, Long GR, Lyon RF, Narris PM, C.R. Steele CR, Hecht-Poinar E (eds) Diversity in auditory mechanics. World Scientific, Singapore, pp. 25–31
Webb JF (1989) Developmental constraints and evolution of the lateral line system in teleost fishes. In: Coombs S, Görner P, Münz H (eds) The mechanosensory lateral line: Neurobiology and evolution. Springer, Berlin Heidelberg New York New York, pp 79–97
Wiersinga-Post JEC, van Netten SM (1998) Amiloride causes changes in the mechanical properties of hair cell bundles in the fish lateral line similar to those induced by dihydrostreptomycin. Proc R Soc Lond B 265:615–623
Wiersinga-Post JEC, van Netten SM (2000) Temperature dependency of cupular mechanics and hair cell frequency selectivity in the fish canal lateral line organ. J Comp Physiol 186: 949–956
Womersley JR (1955) Method for the calculation of velocity, rate of flow and drag in arteries when the pressure gradient is known. J Physiol 127:553–563
Wubbels RJ (1992) Afferent respons of a head canal neuromast of the Ruff (Acerina cernua) lateral line. Comp Biochem Physiol 102A:19–26
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Netten, S.M. Hydrodynamic detection by cupulae in a lateral line canal: functional relations between physics and physiology. Biol Cybern 94, 67–85 (2006). https://doi.org/10.1007/s00422-005-0032-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-005-0032-x