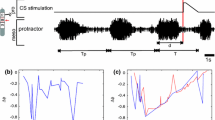
Abstract
Previous authors have proposed two basic hypotheses about the factors that form the basis of locomotor rhythms in walking insects: sensory feedback only or sensory feedback together with rhythmic activity of small neural circuits called central pattern generators (CPGs). Here we focus on the latter. Following this concept, to generate functional outputs, locomotor control must feature both rhythm generation by CPGs at the level of individual joints and coordination of their rhythmic activities, so that all muscles are activated in an appropriate pattern. This work provides an in-depth analysis of an aspect of this coordination process based on an existing network model of stick insect locomotion. Specifically, we consider how the control system for a single joint in the stick insect leg may produce rhythmic output when subjected to ascending sensory signals from other joints in the leg. In this work, the core rhythm generating CPG component of the joint under study is represented by a classical half-center oscillator constrained by a basic set of experimental observations. While the dynamical features of this CPG, including phase transitions by escape and release, are well understood, we provide novel insights about how these transition mechanisms yield entrainment to the incoming sensory signal, how entrainment can be lost under variation of signal strength and period or other perturbations, how entrainment can be restored by modulation of tonic top-down drive levels, and how these factors impact the duty cycle of the motor output.























Similar content being viewed by others
References
Katz PS, Hooper SL (2007) Invertebrate central pattern generators. In: Norrth G, Greenspan RJ (eds) Invertebrate neurobiology. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Marder E, Bucher D (2001) Central pattern generators and the control of rhythmic movements. Curr Biol 11:R986–R996
Marder E, Calabrese RL (1996) Principles of rhythmic motor pattern generation. Physiol Rev 76:687–717
Smith JC, Abdala APL, Borgmann A, Rybak IA, Paton JFR (2013) Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci 36:152–162
Orlovsky GN, Deliagina TG, Grillner S (1999) Neuronal control of locomotion. Oxford University Press, Oxford
Mantziaris C, Bockemühl T, Büschges A (2020) Central pattern generating networks in insect locomotion. Dev Neurobiol 80(1–2):16–30
Büschges A (2020) Connecting the micro with the macro level in motor control: unravelling general sensory influences on leg stepping. J Physiol 597(12):2971–2972
Bidaye SS, Bockemühl T, Büschges A (2018) Six-legged walking in insects: how CPGs, peripheral feedback, and descending signals generate coordinated and adaptive motor rhythms. J Neurophysiol 119:459–475
Bender JA, Simpson EM, Tietz BR, Daltorio KA, Quinn RD, Ritzmann RE (2011) Kinematic and behavioral evidence for a distinction between trotting and ambling gaits in the cockroach Blaberus discoidalis. J Exp Biol 214:2057–2064
Cruse H (1990) What mechanisms coordinate leg movement in walking arthropods. TINS 13:15–21
Grabowska M, Godlewska E, Schmidt J, Daun-Gruhn S (2012) Quadrupedal gaits in hexapod animals-inter-leg coordination in free-walking adult stick insects. J Exp Biol 215:4255–4266
Mendes CS, Bartos I, Akay T, Márka S, Mann RS (2013) Quantification of gait parameters in freely walking wild type and sensory deprived Drosophila melanogaster. Elife 2:e00231
Wendler G (1966) The co-ordination of walking movements in arthropods. Symp Soc Exp Biol 20:229–249
Wosnitza A, Bockemühl T, Dübbert M, Scholz H, Büschges A (2013) Inter-leg coordination in the control of walking speed in Drosophila. J Exp Biol 216:480–491
Schilling M, Cruse H (2020) Decentralized control of insect walking: a simple neural network explains a wide range of behavioral and neurophysiological results. PLoS Comput Biol 16(4):e1007804
Graham D (1985) Influence of coxa-thorax joint receptors on retractor motor output during walking in Carausius morosus. J Exp Biol 114:131–139
Hughes GM (1952) The co-ordination of insect movements. J Exp Biol 29:267–285
Biewener AA (2003) Animal locomotion. Oxford University Press, Oxford
Hooper SL, Guschlbauer Ch, Blümel M, Rosenbaum P, Gruhn M, Akay T, Büschges A (2009) Neural control of unloaded leg posture and leg swing in stick insect, cockroach, and mouse differs from that in larger animals. J Neurosci 29:4109–4119
Büschges A (2005) Sensory control and organization of neural networks mediating coordination of multisegmental organs for locomotion. J Neurophysiol 93:1127–1135
Toth TI, Daun-Gruhn S (2011) A putative neuronal network controlling the activity of the leg motoneurons of the stick insect. NeuroReport 22(18):943–946
Toth TI, Knops S, Daun-Gruhn S (2012) A neuro-mechanical model explaining forward and backward stepping in the stick insect. J Neurophysiol 107(12):3267–80
Toth TI, Grabowska M, Schmidt J, Büschges A, Daun-Gruhn S (2013) A neuro-mechanical model explaining the physiological role of fast and slow muscle fibres at stop and start of stepping of an insect leg. PLoS ONE 8(11):e78246
Knops S, Toth TI, Guschlbauer C, Gruhn M, Daun-Gruhn S (2013) A neuromechanical model for curve walking in the stick insect. J Neurophysiol 109(3):679–691
Toth TI, Daun S (2019) A kinematic model of stick insect walking. Physiol Rep 7(8):e14080
Graham D (1972) An analysis of walking in the first instar and adult stick insect Carausius morosus. J Comput Physiol 81:23–52
Prochazka A (1996) Proprioceptive feedback and movement regulation. In: Rowell L, Sheperd JT (eds) Handbook of physiology. American Physiological Society, New York, pp 89–127
Daun-Gruhn S, Büschges A (2011) From neuron to behavior: dynamic equation-based prediction of biological processes in motor control. Biological Cybernetics 105(1):71–88
Ayali A, Borgmann A, Büschges A, Couzin-Fuchs E, Daun-Gruhn S, Holmes P (2015) The comparative investigation of the stick insect and cockroach models in the study of insect locomotion. Curr Opin Insect Sci 12:1–10
Tóth TI, Grabowska M, Rosjat N, Hellekes K, Borgmann A, Daun-Gruhn S (2015) Investigating inter-segmental connections between thoracic ganglia in the stick insect by means of experimental and simulated phase response curves. Biol Cybern 109(3):349–362
Borgmann A, Toth TI, Gruhn M, Daun-Gruhn S*, Büschges A* (2011) Dominance of local load signals over inter-segmental effects in a motor system. I. Experiments. Biol Cybern 105(5–6): 399—411. *shared senior authorship
Daun-Gruhn S, Toth TI, Borgmann A (2011) Dominance of local load signals over inter-segmental effects in a motor system. II. Simulation studies. Biol Cybern 105(5–6):413–426
Borgmann A, Scharstein H, Büschges A (2007) Intersegmental coordination: influence of a single walking leg on the neighboring segments in the stick insect walking system. J Neurophysiol 98:1685–1696
Borgmann A, Hooper SL, Büschges A (2009) Sensory feedback induced by front-leg stepping entrains the activity of central pattern generators in caudal segments of the stick insect walking system. J Neurosci 29:2972–2983
Daun-Gruhn S (2011) A mathematical modeling study of inter-segmental coordination during stick insect walking. J Comput Neurosci 30(2):255–278
Büschges A (1995) Role of local nonspiking interneurons in the generation of rhythmic motor activity in the stick insect. J Neurobiol 27:488–512
Büschges A, Gruhn M (2008) Mechanosensory feedback in walking: from joint control to locomotory patterns. Adv Insect Physiol 34:194–234
Wang X, Rinzel J (1992) Alternating and synchronous rhythms in reciprocally inhibitory model neurons. Neural Comput 4:84–97
Skinner F, Kopell N, Marder E (1994) Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. J Comput Neurosci 1:69–87
Daun S, Rubin J, Rybak I (2009) Control of oscillation periods and phase durations in half-center central pattern generators: a comparative mechanistic analysis. J Comput Neurosci 27(1):3–36
Rosenbaum P, Schmitz J, Schmidt J, Büschges A (2015) Task-dependent modification of leg motor neuron synaptic input underlying changes in walking direction and walking speed. J Neurophysiol 114:1090–1101
Mentel T, Weiler V, Büschges A, Pflüger H-J (2008) Activity of neuromodulatory neurones during stepping of a single insect leg. J Insect Physiol 54(1):51–61
Stolz T, Diesner M, Neupert S, Hess ME, Delgado-Betancourt E, Pflüger H-J, Schmidt J (2019) Descending octopaminergic neurons modulate sensory evoked activity of thoracic motor neurons in stick insects. J Neurophysiol 122:2388–2413
Hooper SL, Büschges A (2017) Neurobiology of motor contro—fundamental concepts and new directions. In: SL Hooper, ABüschges (eds) Wiley Blackwell
Danner SM, Shevtsova NA, Frigon A, Rybak IA (2017) Computational modeling of spinal circuits controlling limb coordination and gaits in quadrupeds. eLife 6:e31050
Ekeberg Ö, Pearson KG (2005) Computer simulation of stepping in the hind legs of the cat: an examination of the mechanisms regulating the stance-to-swing transition. J Neurophysiol 94:4256–4268
Yeldesbay A, Toth TI, Daun S (2018) The role of phase shifts of sensory inputs in walking revealed by means of phase reduction. J Comput Neurosci 44(1):313–339
Holmes PJ, Full RJ, Koditschek D, Guckenheimer J (2006) The dynamics of legged locomtion: models, analysis, and challenges. SIAM Rev 48(2):207–304
Sponberg S, Full RJ (2008) Neuromechanical response of muscoskeletal structures in cockroaches during rapid running on rough terrain. J Exp Biol 211:446
Fuchs E, Holmes P, David I, Ayali A (2012) Proprioceptive feedback reinforces centrally generated stepping patterns in the cockroach. J Exp Biol 215:1884–1891
Gruhn M, Zehl L, Büschges A (2009) Straight walking and turning on the slippery surface. J Exp Biol 212:194–209
Bidaye SS, Machacek C, Wu Y, Dickson BJ (2014) Neuronal control of Drosophila walking direction. Science 344:97–101
Somers D, Kopell N (1993) Rapid synchronization through fast threshold modulation. Biol Cybern 68:393–407
Rubin J, Terman D (2002) Geometric singular perturbation analysis of neuronal dynamics. In: Fiedler B (ed) Handbook of dynamical systems, vol 2. Elsevier, Amsterdam, pp 93–146
Ghigliazza R, Holmes P (2004) A minimal model of a central pattern generator and motoneurons for insect locomotion. SIAM J Appl Dyn Syst 3:671–700
Zhang C, Lewis T (2013) Phase response properties of half-center oscillators. J Comput Neurosci 35:55–74
Aminzare Z, Holmes Srivastava V (2018) Gait transitions in a phase oscillator model of an insect central pattern generator. SIAM J Appl Dyn Syst 1:626–671
Acknowledgements
This work was partially supported by NSF DMS awards 1612913 and 1951095 (JR). SD gratefully acknowledges support from the German Research Foundation (DA1953/5-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Benjamin Lindner.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Codianni, M.G., Daun, S. & Rubin, J.E. The roles of ascending sensory signals and top-down central control in the entrainment of a locomotor CPG. Biol Cybern 114, 533–555 (2020). https://doi.org/10.1007/s00422-020-00852-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00422-020-00852-8