Abstract
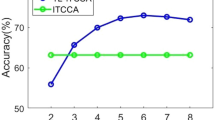
A method that has recently been mentioned as information encoding brain is cross-frequency coupling (CFC). It is generally assumed that CFC can play a crucial role in perception, memory, and attention. In this study, two new indices for evaluating frequency–amplitude coupling (FAC) through generalized linear model (GLM) and linear regression method were introduced and investigated along with other CFC indices. Electroencephalogram (EEG) signals were recorded during covert visual attention tasks to find out the CFC index capability so as to distinguish different states in the mentioned tasks. To this end, machine learning algorithms were used and four various types of CFC, phase–amplitude coupling (PAC), phase–phase coupling (PPC), amplitude–amplitude coupling (AAC), and frequency–amplitude coupling (FAC) in recorded signals were considered as inputs for classifiers. The results demonstrated that the proposed method used for evaluating FAC through linear regression can provide more information about the different states in two covert attention tasks using quadratic discriminant analysis (QDA) by classification performance of 94.21% and 90.54% in color and direction tasks, respectively. Also, FAC that used a GLM model and PAC had a higher performance compared with PPC and AAC in color task (90.74 and 92.24% against 83.21 and 86.22). We can conclude that CFC can encompass useful information about semantic category of stimuli in covert attention tasks and can be used as an acceptable alternative for the time–frequency features of brain signals.










Similar content being viewed by others
References
Carrasco M (2011) Visual attention: the past 25 years. Vision Res 51(13):1484–1525
Ling S, Liu T, Carrasco M (2009) How spatial and feature-based attention affect the gain and tuning of population responses. Vis Res 49(10):1194–1204
Baldassi S, Verghese P (2005) Attention to locations and features: different top-down modulation of detector weights. J Vis 5(6):7–7
Boynton GM (2009) A framework for describing the effects of attention on visual responses. Vision Res 49(10):1129–1143
Maunsell JH, Treue S (2006) Feature-based attention in visual cortex. Trends Neurosci 29(6):317–322
Serences JT, Boynton GM (2007) Feature-based attentional modulations in the absence of direct visual stimulation. Neuron 55(2):301–312
Ahmadi A, Davoudi S, Daliri MR (2019) Computer aided diagnosis system for multiple sclerosis disease based on phase to amplitude coupling in covert visual attention. Comput Methods Programs Biomed 169:9–18
Carrasco M (2006) Covert attention increases contrast sensitivity: psychophysical, neurophysiological and neuroimaging studies. Prog Brain Res 154:33–70
Eckstein MP (2004) Active vision: the psychology of looking and seeing. SAGE Publications Sage UK, London
Proverbio AM, Del Zotto M, Zani A (2007) The emergence of semantic categorization in early visual processing: ERP indices of animal vs. artifact recognition. BMC neuroscience 8(1):24
Paz-Caballero D, Cuetos F, Dobarro A (2006) Electrophysiological evidence for a natural/artifactual dissociation. Brain Res 1067(1):189–200
Adorni R, Proverbio AM (2009) New insights into name category-related effects: is the age of acquisition a possible factor? Behav Brain Funct 5(1):33
Buzsaki G (2006) Rhythms of the brain. Oxford University Press, Oxford
Jirsa V, Müller V (2013) Cross-frequency coupling in real and virtual brain networks. Front Comput Neurosci 7:78
Jafakesh S, Jahromy FZ, Daliri MR (2016) Decoding of object categories from brain signals using cross frequency coupling methods. Biomed Signal Process Control 27:60–67
Kramer M, Eden U (2013) Assessment of cross-frequency coupling with confidence using generalized linear models. J Neurosci Methods 220(1):64–74
Canolty RT, Knight RT (2010) The functional role of cross-frequency coupling. Trends Cogn Sci 14(11):506–515
van Wijk B et al (2015) Parametric estimation of cross-frequency coupling. J Neurosci Methods 243:94–102
Alegre M (2016) Cross-frequency coupling in the pathophysiology of Parkinson’s disease. Clin Neurophysiol 127(3):e29
Liu Y et al (2018) Epileptic seizure detection from EEG signals with phase–amplitude cross-frequency coupling and support vector machine. Int J Mod Phys B 32(08):1850086
Jacobs D et al (2018) Classification of pre-clinical seizure states using scalp EEG cross-frequency coupling features. IEEE Trans Biomed Eng 65(11):2440–2449
Voytek B et al (2013) A method for event-related phase/amplitude coupling. Neuroimage 64:416–424
Tort AB et al (2008) Dynamic cross-frequency couplings of local field potential oscillations in rat striatum and hippocampus during performance of a T-maze task. Proc Natl Acad Sci 105(51):20517–20522
Esghaei M, Daliri MR, Treue S (2015) Attention decreases phase-amplitude coupling, enhancing stimulus discriminability in cortical area MT. Front Neural Circuits 9:82
Sauseng P et al (2015) Predictive coding in visual search as revealed by cross-frequency EEG phase synchronization. Front Psychol 6:1655
Lisman JE, Jensen O (2013) The theta-gamma neural code. Neuron 77(6):1002–1016
Voytek B et al (2010) Shifts in gamma phase–amplitude coupling frequency from theta to alpha over posterior cortex during visual tasks. Front Human Neurosci 4:191
Tort AB et al (2010) Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104(2):1195–1210
FitzGerald TH et al (2013) Cross-frequency coupling within and between the human thalamus and neocortex. Front Human Neurosci 7:84
Ahmadi A, et al. (2017) Phase and amplitude coupling feature extraction and recognition of Ictal EEG using VMD. In: 2017 IEEE 4th international conference on knowledge-based engineering and innovation (KBEI). 2017. IEEE
Ahmadi A, et al. (2018) Classification of epileptic EEG signals by wavelet based CFC. In: 2018 Electric electronics, computer science, biomedical engineerings’ meeting (EBBT). 2018. IEEE
Schutter DJ, Knyazev GG (2012) Cross-frequency coupling of brain oscillations in studying motivation and emotion. Motiv Emot 36(1):46–54
Canolty RT et al (2006) High gamma power is phase-locked to theta oscillations in human neocortex. Science 313(5793):1626–1628
Lachaux J-P et al (1999) Measuring phase synchrony in brain signals. Hum Brain Mapp 8(4):194–208
Vanhatalo S et al (2004) Infraslow oscillations modulate excitability and interictal epileptic activity in the human cortex during sleep. Proc Natl Acad Sci USA 101(14):5053–5057
Mormann F et al (2005) Phase/amplitude reset and theta–gamma interaction in the human medial temporal lobe during a continuous word recognition memory task. Hippocampus 15(7):890–900
Bruns A, Eckhorn R (2004) Task-related coupling from high-to low-frequency signals among visual cortical areas in human subdural recordings. Int J Psychophysiol 51(2):97–116
Cohen MX (2008) Assessing transient cross-frequency coupling in EEG data. J Neurosci Methods 168(2):494–499
Penny W et al (2008) Testing for nested oscillation. J Neurosci Methods 174(1):50–61
Chehelcheraghi M et al (2017) A neural mass model of cross frequency coupling. PLoS ONE 12(4):e0173776
Witte H et al (2008) Analysis and modeling of time-variant amplitude–frequency couplings of and between oscillations of EEG bursts. Biol Cybern 99(2):139–157
Witte H et al (2011) Time-variant analysis of phase couplings and amplitude–frequency dependencies of and between frequency components of EEG burst patterns in full-term newborns. Clin Neurophysiol 122(2):253–266
Gu Y et al (2009) Offline identification of imagined speed of wrist movements in paralyzed ALS patients from single-trial EEG. Front Neurosci 3:3
Rafiee J et al (2011) Wavelet basis functions in biomedical signal processing. Expert Syst Appl 38(5):6190–6201
Ince NF, Tewfik A, Arica S (2005) Classification of movement EEG with local discriminant bases. In: acoustics, speech, and signal processing, 2005. proceedings.(ICASSP’05). IEEE International conference on. 2005. IEEE
Lal TN et al (2004) Support vector channel selection in BCI. IEEE Trans Biomed Eng 51(6):1003–1010
Palaniappan R et al (2002) A new brain-computer interface design using fuzzy ARTMAP. IEEE Trans Neural Syst Rehabil Eng 10(3):140–148
Mookiah MRK et al (2012) Data mining technique for automated diagnosis of glaucoma using higher order spectra and wavelet energy features. Knowl-Based Syst 33:73–82
Thiery T et al (2016) Decoding the locus of covert visuospatial attention from EEG signals. PLoS ONE 8:e0160304
Zhang D et al (2010) An independent brain–computer interface using covert non-spatial visual selective attention. J Neural Eng 7(1):016010
Andersson P et al (2012) Real-time decoding of the direction of covert visuospatial attention. J Neural Eng 9(4):045004
Alpaydin E (2014) Introduction to machine learning. MIT press, Cambridge
D’Andrea A et al (2019) Alpha and alpha-beta phase synchronization mediate the recruitment of the visuospatial attention network through the superior longitudinal fasciculus. NeuroImage 188:722–732
Márton CD et al (2019) Signature patterns for top-down and bottom-up information processing via cross-frequency coupling in macaque auditory cortex. eNeuro 6(2):ENEURO.0467
Dvorak D, Fenton AA (2014) Toward a proper estimation of phase–amplitude coupling in neural oscillations. J Neurosci Methods 225:42–56
Aru J et al (2015) Untangling cross-frequency coupling in neuroscience. Curr Opin Neurobiol 31:51–61
Alan VO, Ronald WS, John R (1989) Discrete-time signal processing. Printice Hall Inc, New Jersey
Tass P et al (1998) Detection of n: m phase locking from noisy data: application to magnetoencephalography. Phys Rev Lett 81(15):3291
Sinkkonen J, Tiitinen H, Näätänen R (1995) Gabor filters: an informative way for analysing event-related brain activity. J Neurosci Methods 56(1):99–104
Penny WD et al (2011) Statistical parametric mapping: the analysis of functional brain images. Academic press, Cambridge
Berman JI et al (2012) Variable bandwidth filtering for improved sensitivity of cross-frequency coupling metrics. Brain Connect 2(3):155–163
Acharya UR et al (2015) Computer-aided diagnosis of diabetic subjects by heart rate variability signals using discrete wavelet transform method. Knowl-Based Syst 81:56–64
Azarmi F et al (2019) Granger causality analysis in combination with directed network measures for classification of MS patients and healthy controls using task-related fMRI. Comput Biol Med 115:103495
Yang S et al (2020) Selection of features for patient-independent detection of seizure events using scalp EEG signals. Comput Biol Med 119:103671
Behroozi M, Daliri MR, Shekarchi B (2016) EEG phase patterns reflect the representation of semantic categories of objects. Med Biol Eng Compu 54(1):205–221
Mardi Z, Ashtiani SNM, Mikaili M (2011) EEG-based drowsiness detection for safe driving using chaotic features and statistical tests. J Med signals Sens 1(2):130
Ahmadi A, Shalchyan V, Daliri MR (2017) A new method for epileptic seizure classification in EEG using adapted wavelet packets. In: Electric electronics, computer science, biomedical engineerings’ meeting (EBBT), 2017.IEEE
Sargezeh BA, Tavakoli N, Daliri MR (2019) Gender-based eye movement differences in passive indoor picture viewing: an eye-tracking study. Physiol Behav 206:43–50
Huang G-B, Zhu Q-Y, Siew C-K (2006) Extreme learning machine: theory and applications. Neurocomputing 70(1):489–501
Daliri MR (2012) A hybrid automatic system for the diagnosis of lung cancer based on genetic algorithm and fuzzy extreme learning machines. J Med Syst 36(2):1001–1005
Daliri MR (2015) Combining extreme learning machines using support vector machines for breast tissue classification. Comput Methods Biomechan Biomed Eng 18(2):185–191
Srivastava S, Gupta MR, Frigyik BA (2007) Bayesian quadratic discriminant analysis. J Mach Learn Res 8:1277–1305
Jensen O, Kaiser J, Lachaux J-P (2007) Human gamma-frequency oscillations associated with attention and memory. Trends Neurosci 30(7):317–324
Nyhus E, Curran T (2010) Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev 34(7):1023–1035
Palanca BJ, DeAngelis GC (2005) Does neuronal synchrony underlie visual feature grouping? Neuron 46(2):333–346
Hülsemann MJ, Naumann E, Rasch B (2019) Quantification of phase-amplitude coupling in neuronal oscillations: comparison of phase-locking value, mean vector length, modulation index, and generalized linear modeling cross-frequency coupling. Front Human Neurosci 13:573
Trachel RE, Clerc M, Brochier TG (2015) Decoding covert shifts of attention induced by ambiguous visuospatial cues. Front Human Neurosci 9:358
van Schouwenburg MR, den Ouden HE, Cools R (2015) Selective attentional enhancement and inhibition of fronto-posterior connectivity by the basal ganglia during attention switching. Cereb Cortex 25(6):1527–1534
Foster JJ et al (2017) Alpha-band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychol Sci 28(7):929–941
Treder MS, Blankertz B (2010) (C) overt attention and visual speller design in an ERP-based brain-computer interface. Behav Brain Funct 6(1):28
Treder MS et al (2011) Brain-computer interfacing using modulations of alpha activity induced by covert shifts of attention. J Neuroeng Rehabil 8(1):24
Liu Y, Zhou Z, Hu D (2011) Gaze independent brain–computer speller with covert visual search tasks. Clin Neurophysiol 122(6):1127–1136
Reichert C et al (2017) A comparative study on the detection of covert attention in event-related EEG and MEG signals to control a BCI. Front Neurosci 11:575
Friese U et al (2013) Successful memory encoding is associated with increased cross-frequency coupling between frontal theta and posterior gamma oscillations in human scalp-recorded EEG. Neuroimage 66:642–647
Kane MJ, Engle RW (2002) The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev 9(4):637–671
Ranganath C (2006) Working memory for visual objects: complementary roles of inferior temporal, medial temporal, and prefrontal cortex. Neuroscience 139(1):277–289
Zanto TP et al (2011) Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nat Neurosci 14(5):656–661
Axmacher N et al (2010) Cross-frequency coupling supports multi-item working memory in the human hippocampus. Proc Natl Acad Sci 107(7):3228–3233
Händel B, Haarmeier T (2009) Cross-frequency coupling of brain oscillations indicates the success in visual motion discrimination. Neuroimage 45(3):1040–1046
Munia TT, Aviyente S (2019) Time-frequency based phase-amplitude coupling measure for neuronal oscillations. Sci Rep 9(1):1–15
Siems M, Siegel M (2019) Dissociated cortical phase-and amplitude-coupling patterns in the human brain. BioRxiv, 2019: p. 485599
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Davoudi, S., Ahmadi, A. & Daliri, M.R. Frequency–amplitude coupling: a new approach for decoding of attended features in covert visual attention task. Neural Comput & Applic 33, 3487–3502 (2021). https://doi.org/10.1007/s00521-020-05222-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00521-020-05222-w