Abstract
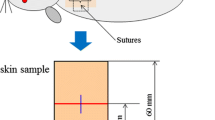
There are many methods of healing wounds. Among these, light therapy is reported to be beneficial, as beams assist the human body in treating, sterilizing, and regenerating cells. Both Laser and LED irradiation with specific wavelengths induce proliferation of fibroblasts depending on the wound area or wavelength and are effective in wound healing. This study used 8-week-old 250–300 g Male Sprague–Dawley Rats (ILAR Code: NTacSam:SD). The experiment was carried out for the non-irradiation group and the Red–Green–Blue LEDs irradiation groups (n of each group = 5). The experiment animals were relieved for 24 h after wounds had been excised and then the Red–Green–Blue LEDs irradiation groups were given irradiation therapy over 9 days 1 h per day. Immunohistochemical staining was conducted for cytokeratin in order to precisely measure the defect size. Also, Masson’s trichrome staining was conducted for comparison of collagen between the Red–Green–Blue LEDs irradiation groups and the non-irradiation group. Animals treated with Blue LEDs irradiation (p < 0.05), Green LEDs irradiation (p < 0.05), and Red LEDs irradiation (N.S.) healed at a faster rate than non-irradiated group. The LEDs irradiated groups also had more collagen, according to Masson’s trichrome staining for collagen analysis. The Red–Green–Blue LEDs irradiation had a beneficial effect on wound healing and could probably replace low power laser treatment.




Similar content being viewed by others
References
Cheon MW, Park YP (2010) Wound healing effect of 525 nm Green LED irradiation on skin wounds of male sprague dawley rats. Trasn EEM 11(5):226–229
Pinaki S, Amrita S (2011) Security enhanced communication in wireless sensor networks using reed-muller codes and partially balanced incomplete block designs. J Conv 2:23–30
Wauyo AB, Pek I, Chen X, Yeoh WS (2009) Design and evaluation of lightweight middleware for personal wireless body area network. Personal Ubiquitous Comput 13(7):509–525
Vassis D, Belsis P, Skourlas C, Pantziou G (2010) Providing advanced remote medical treatment services through pervasive environments. Personal Ubiquitous Comput 14(6):563–573
Park HS, Kim BS, Kim TH, Choi M, Kim KH (2010) The principles of intense pulsed light and its clinical application. Kor J Dermatol 45(9):735–740
Shin MH, Rhie GE, Park CH, Kim KH, Cho KH, Eun HC, Chung JH (2005) Modulation of collagen metabolism by the topical application of dehydroepiandrosterone to human skin. J Invest Dermatol 124(2):315–323
Carmichael J, Degraff WG, Gazdar AF, Minna JD, Mitchell JB (1987) Evaluation of a tetrazolium-based semiautomated colorimetraic assay: assessment of chemosensitivity testing. Cancer Res 47(15):936–942
Mullins F, Minton JP, Hove RC, Dearman JR, Mcknight WB (1996) The effects of high energy laser pulses on the primate liver. Surg Gynecol Obstet 122:727–732
Polanyi TG, Bredemeier HC, Davis TW (1970) A CO2 laser for surgical research. Med Biol Engng 8(6):541–548
Kirsch AJ, Miller MI, Hensle TW, Chang DT, Shabsigh R, Olsson CA, Connor JP (1995) Laser tissue soldering in urinary tract reconstruction: first human experience. Urology 49(2):261–266
Beauvoit B, Evans SM, Jenkins TW (1995) Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal Biochem 226(1):167–174
Beauvoit B, Kitai T, Chance B (1994) Contribution of the mitochondrial compartment to the optical porperties of the rat liver: a theoretical and practical approach. Biophys J 226(6):167–174
Bisht D, Gupta SC, Misra V, Mital VP, Sharma P (1994) Effect of low intensity laser radiation on healing of open skin woulds in rats. Indian J Med Res 100:43–46
Sakurai Y, Yamaguchi M, Abiko Y (2000) Inhibitory effect of low-level laser irradiation on LPS-stimulated prostaglandin E2 production and cyclooxygenase-2 in human gingival fibroblasts. Eur J Oral Sci 108(1):29–34
Whelan HT, Smits BLJ, Buchman EV, Whelan NT, Turner SG, Margolis DA, Cevenini V, Stinson H, Ignatius R, Martin T, Cwiklinski J, Philippi AF, Graf WR, Hodgson BGL, Kane M, Chen G, Caviness J (2001) Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg 19(6):305–314
Whelan HT, Buchmann EV, Dhokalia A, Kane MP, Whelan NT, Wong-Riley MTT, Eells JT, Gould LJ, Hammamieh R, Das R, Jett M (2004) Effect of NASA light-emitting diode irradiation on molecular changes for wound healing in diabetic mice. J Clin Laser Med Surg 21(2):67–74
Wong-Riley MTT, Bai XB, Buchamann E, Whelan HT (2001) Light-emitting diode treatment reverses the effect of TTX on cytochrome oxidase in neurons. NeuroReport 12(14):3033–3037
MAlena RAP, Livia SP, Silvia RAR, Zilton AA (2003) Influence of low level laser therapy on wound healing and its biological action upon myofibroblasts. Lasers Surg Med 32(3):239–244
Braekt MMH, Alphen FAM, Maltha JC (1991) Effect of low level laser therapy on wound healing after palatal surgery in beagle dogs. Lasers Surg Med 11(5):462–470
Imanishia J, Kamiyamaa K, Iguchi I, Kita M, Sotozono C, Kinoshita S (2000) Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res 19(1):113–129
Kana JS, Hutschenreiter G (1981) Effect of low-power density laser radiation on healing of open skin wounds in rats. Arch Surg 116:293–296
Vinck EM, Cagnie BJ, Cornelissen MJ, Declercq HA, Cambier DC (2003) Increased fibroblast proliferation induced by light emitting diode and low power laser irradiation. Laser Med sci 18(2):95–99
Cheon MW (2008) Effect of 8 mW 525 nm LEDs Light Irradiation on the defect reduction in the skin wound of SD-rat. Trans EEM 9(3):116–119
Chance B, Nioka S, Kent J, McCully K, Fountain M, Greenfeld R, Holtom G (1988) Time-resolved spectroscopy of hemoglobin and myoglobin in resting and ischemic muscle. Anal Biochem 174(2):698–707
Barnadas MA, Brunet S, Sureda A, López R, Curell R, Sierra J, Alomar A, Spain B (1999) Exuberant granulation tissue associated with chronic graft-versus-host disease after transplantation of peripheral blood progenitor cells. J Am Acad Dermatol 41(5):876–879
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cheon, M.W., Kim, T.G., Lee, Y.S. et al. Low level light therapy by Red–Green–Blue LEDs improves healing in an excision model of Sprague–Dawley rats. Pers Ubiquit Comput 17, 1421–1428 (2013). https://doi.org/10.1007/s00779-012-0577-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00779-012-0577-3