Abstract
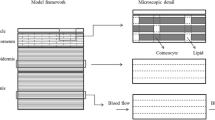
In this paper we present a mathematical diffusion model describing the transient transdermal penetration of two non-volatile substances, the lipophilic flufenamic acid and the hydrophilic caffeine, after finite dosing in an aqueous vehicle system. A striking feature of this microscopic diffusion model is its ability to predict concentration-depth profiles. Relevant input parameters are obtained from a previously published infinite dose study (Naegel et al in Eur J Pharm Biopharm 68:368–379, 2008; Hansen et al in Eur J Pharm Biopharm 68:352–367, 2008). The quality of the model has been evaluated by comparing the concentration-depth profiles in stratum corneum (SC) and deeper skin layers of the experiment with those of the simulation. The results from the experiment and the simulation are in good agreement. The study addresses benefits and shortcomings of the model, and discusses future perspectives such as incorporating different morphological regions of the SC.
Similar content being viewed by others
References
Mitragotri S., Anissimov Y.G., Bunge A.L., Frasch H.F., Guy R.H., Hadgraft J., Kasting G.B., Lane M.E., Roberts M.S.: Mathematical models of skin permeability: an overview. Int. J. Pharm. 418, 115–129 (2011)
OECD Guideline for the Testing of Chemicals. Skin Absorption: in vitro Method, 428 (2004)
OECD Guidance Document for the Conduct of Skin Absorption Studies. OECD series on testing and assessment, no. 28 (2004)
Bhatt P.P., Hanna M.S., Szeptycki P., Higuchi T.: Finite dose transport of drugs in liquid formulations through stratum corneum: analytical solution to a diffusion model. Int. J. Pharm. 50, 197–203 (1989)
Kubota K.: Finite dose percutaneous drug absorption: a BASIC program for the solution of the diffusion equation. Comp. Biomed. Res. 24, 196–207 (1991)
Seta Y., Ghanem A.H., Higuchi W.I., Borsadia S., Behl C.R., Malick A.W.: Physical model approach to understanding finite dose transport and uptake of hydrocortisone in hairless guinea-pig skin. Int. J. Pharm. 81, 89–99 (1992)
Anissimov Y.G., Roberts M.S.: Diffusion modeling of percutaneous absorption kinetics: 2. Finite vehicle volume and solvent deposited solids. J. Pharm. Sci. 90, 504–520 (2001)
Kasting G.B.: Kinetics of finite dose absorption through skin 1. Vanillylnonanamide. J. Pharm. Sci. 90, 202–212 (2001)
Kasting G.B., Miller M.A.: Kinetics of finite dose absorption through skin 2. Volatile compounds. J. Pharm. Sci. 95, 268–280 (2006)
Miller M.A., Bhatt V., Kasting G.B.: Absorption and evaporation of benzyl alcohol from skin. J. Pharm. Sci. 95, 281–291 (2006)
Kretsos K., Kasting G.B., Nitsche J.M.: Distributed diffusion—clearance model for transient drug distribution within the skin. J. Pharm. Sci. 93, 2820–2835 (2004)
Kasting G.B., Miller M.A., Bhatt V.D.: A spreadsheet-based method for estimating the skin disposition of volatile compounds: application to n,n-diethyl-m-toluamide (DEET). J. Occup. Env. Hyg. 5, 633–644 (2008)
Nitsche J.M., Kasting G.B.: Biophysical models for skin transport and absorption. In: Roberts, M.S., Walters, K.A. (eds) Dermal Absorption and Toxicity Assessment, 2nd edn, pp. 249–267. Informa Healthcare USA, New York (2008)
Kasting G.B., Miller M.A., Nitsche J.M.: Absorption and evaporation of volatile compounds applied to skin. In: Walters, K.A., Roberts, M.S. (eds) Dermatologic, Cosmeceutic and Cosmetic Development, pp. 385–400. Informa Healthcare USA, New York (2008)
Naegel A., Hansen S., Neumann D., Lehr C.M., Schaefer U.F., Wittum G., Heisig M.: In-silico model of skin penetration based on experimentally determined input parameters. Part II: mathematical modelling of in-vitro diffusion experiments. Identification of critical input parameters. Eur. J. Pharm. Biopharm. 68, 368– 379 (2008)
Hansen S., Henning A., Naegel A., Heisig M., Wittum G., Neumann D., Kostka K.-H., Zbytovska J., Lehr C.M., Schaefer U.F.: In-silico model of skin penetration based on experimentally determined input parameters. Part I: Experimental determination of partition and diffusion coefficients. Eur. J. Pharm. Biopharm. 68, 352–367 (2008)
Naegel A., Heisig M., Wittum G.: Computational Modeling of the skin barrier. Methods Mol. Biol. 763, 1–32 (2011)
Selzer, D., Hahn, T., Naegel, A., Heisig, M., Kostka, K.H., Lehr, C.M., Neumann, D., Schäfer, U.F., Wittum, G.: Finite dose skin mass balance including the lateral part—comparison between experiment, pharmacokinetic modeling, and diffusion models. J. Contr. Rel. (2012, to appear)
Wagner H., Kostka K.H., Lehr C.M., Schaefer U.F.: Drug distribution in human skin using two different in vitro test systems: comparison with in vivo data. Pharm. Res. 17, 1475–1481 (2000)
Idson B.: Percutaneous absorption. J. Pharm. Sci. 64, 901–924 (1975)
King C.S., Barton S.P., Nicholls S., Marks R.: The change in stratum corneum as a function of depth. Br. J. Dermatol. 100, 165–172 (1979)
Anissimov Y.G., Roberts M.S.: Diffusion modeling of percutaneous absorption kinetics: 4. Effects of a slow equilibration process within stratum corneum on absorption and desorption kinetics. J. Pharm. Sci. 98, 772–781 (2009)
Nitsche J.M., Frasch H.F.: Dynamics of diffusion with reversible binding in microscopically heterogeneous membranes: general theory and applications to dermal penetration. Chem. Eng. Sci. 66, 2019–2026 (2011)
Frasch H.F., Barbero A.M., Hettick J.M., Nitsche J.M.: Tissue binding affects the kinetics of theophylline diffusion through the stratum corneum barrier layer of skin. J. Pharm. Sci. 100, 2989–2995 (2011)
Hahn T., Hansen S., Neumann D., Kostka K.H., Lehr C.M., Muys L., Schaefer U.F.: Infrared densitometry: a fast and non-destructive method for exact stratum corneum depth calculation for in vitro tape-stripping. Skin Pharm. Physiol. 23, 183–192 (2010)
Melero A., Hahn T., Schaefer U.F., Schneider M.: In vitro human skin segmentation and drug concentration-skin depth profiles. Methods Mol. Biol. 763, 33–50 (2011)
Naegel, A., Heisig, M., Wittum, G.: Detailed modelling of skin penetration—an overview. Adv. (2012, submitted)
Cooper E.R., Berner B.: Finite dose pharmacokinetics of skin penetration. J. Pharm. Sci. 74, 1100–1102 (1985)
Kubota K., Maibach H.I.: Estimation of the permeability coefficient from a finite-dose, in vitro percutaneous drug permeation study. J. Pharm. Sci. 80, 1001–1002 (1991)
Kubota K., Yamada T.: Finite dose percutaneous drug absorption: theory and its application to in vitro timolol permeation. J. Pharm. Sci. 79, 1015–1019 (1990)
Kubota K., Koyama E., Yasuda K.: A random walk method for percutaneous drug absorption pharmacokinetics: application to repeated administration of a therapeutic timolol patch. J. Pharm. Sci. 80, 752–756 (1991)
Kubota K., Twizell E.H., Maibach H.I.: Drug release from a suspension with a finite dissolution rate: theory and its application to a betamethasone 17-valerate patch. J. Pharm. Sci. 83, 1593–1599 (1994)
Krüse J., Golden D., Wilkinson S., Williams F., Kezic S., Corish J.: Analysis, interpretation, and extrapolation of dermal permeation data using diffusion-based mathematical models. J. Pharm. Sci. 96, 682–703 (2007)
Bunge A.L.: Estimating Dermal Absorption from Finite Volume Exposures. OEESC 2007, Golden CO (2007)
Buist H.E., van Burgsteden J.A., Freidig A.P., Maas W.J.M., van de Sandt J.J.M.: New in vitro dermal absorption database and the prediction of dermal absorption under finite conditions for risk assessment purposes. Regul. Toxicol. Pharmacol. 57, 200–209 (2010)
Davies M., Pendlington R.U., Page L., Roper C.S., Sanders D.J., Bourner C., Pease C.K., MacKay C.: Determining epidermal disposition kinetics for use in an integrated nonanimal approach to skin sensitization risk assessment. Tox. Sci. 119(2), 308–318 (2011)
Elias P.M., Menon G.K., Grayson S., Brown B.E.: Membrane structural alterations in murine stratum corneum: relationship to the localization of polar lipids and phospholipases. J. Invest. Dermatol. 91, 3–10 (1988)
Mueller B., Anissimov Y.G., Roberts M.S.: Unexpected clobetasol propionate profile in human stratum corneum after topical application in vitro. Pharm. Res. 20, 1835–1837 (2003)
Melero A., Garrigues T.M., Alos M., Kostka K.H., Lehr C.M., Schaefer U.F.: Nortriptyline for smoking cessation: release and human skin diffusion from patches. Int. J. Pharm. 378, 101–107 (2009)
Roberts M.S., Cross S.E., Anissimov Y.G.: Factors affecting the formation of a skin reservoir for topically applied solutes. Skin Pharmacol. Physiol. 17, 3–16 (2004)
Richter T., Peuckert C., Sattler M., Koenig K., Riemann I., Hintze U., Wittern K.-P., Wiesendanger R., Wepf R.: Dead but highly dynamic—the stratum corneum is divided into three hydration zones. Skin Pharmacol. Physiol. 17(5), 246–257 (2004)
Chapman S.J., Walsh A., Jackson S.M., Friedmann P.S.: Lipids, proteins and corneocyte adhesion. Arch. Dermatol. Res. 283, 167–173 (1991)
Bonte F., Saunois A., Pinguet P., Meybeck A.: Existence of a lipid gradient in the upper stratum corneum and its possible biological significance. Arch. Dermatol. Res. 289, 78–82 (1997)
Weerheim A., Ponec M.: Determination of stratum corneum lipid profile by tape stripping in combination with high-performance thin-layer chromatography. Arch. Dermatol. Res. 293, 191–199 (2001)
Anissimov Y.G., Roberts M.S.: Diffusion modeling of percutaneous absorption kinetics: 3. Variable diffusion and partition coefficients, consequences for stratum corneum depth profiles and desorption kinetics. J. Pharm. Sci. 93, 470–487 (2004)
Chaudhuri S.R., Kasting G.B., Krantz W.B.: Percutaneous absorption of volatile solvents following transient liquid exposures: I. Model development. Chem. Eng. Sci. 64(5), 1027–1035 (2009)
Chambin-Remoussenard O., Treffel P., Bechtel Y., Agache P.: Surface recovery and stripping methods to quantify percutaneous absorption of caffeine in humans. J. Pharm. Sci. 11, 1099–1101 (1982)
Potard G., Laugel C., Schaefer H., Marty J.-P.: The stripping technique: in vitro absorption and penetration of five UV filters on excised fresh human skin. Skin Pharmacol. Appl. Skin Physiol. 13, 336–344 (2000)
Potard G., Laugel C., Baillet A., Schaefer H., Marty J.P.: Quantitative HPLC analysis of sunscreens and caffeine during in vitro percutaneous penetration studies. Int. J. Pharm. 189, 249–260 (1999)
Wagner H., Kostka K.H., Lehr C.M., Schaefer U.F.: Interrelation of permeation and penetration parameters obtained from in vitro experiments with human skin and skin equivalents. J. Contr. Rel. 75, 283–295 (2001)
Ben Mustapha R., Lafforgue C., Fenina N., Marty J.P.: Influence of drug concentration on the diffusion parameters of caffeine. Indian J. Pharmacol. 43(2), 157–162 (2011)
Author information
Authors and Affiliations
Corresponding author
Additional information
Arne Naegel, Tsambika Hahn contributed equally to this work.
Rights and permissions
About this article
Cite this article
Naegel, A., Hahn, T., Schaefer, U.F. et al. Finite dose skin penetration: a comparison of concentration-depth profiles from experiment and simulation. Comput. Visual Sci. 14, 327–339 (2011). https://doi.org/10.1007/s00791-012-0186-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00791-012-0186-8