Abstract
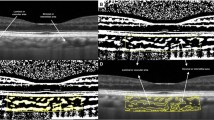
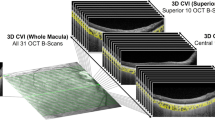
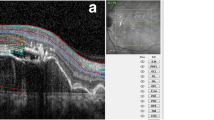
Retinitis pigmentosa (RP) is a group of genetic disorders, characterized by degeneration of photoreceptor cells which is the main cause of choroidal thinning. It is one of the leading causes of blindness worldwide. Thus, an investigation of choroidal changes is required for a better understanding of disease and diagnosis of RP. In this paper, we propose an automatic technique for measuring the choroidal parameters in optical coherence tomography (OCT) images of eyes with RP. The parameters include the total choroidal area (TCA), luminal area (LA), stromal area (SA), and choroidal thickness (CT). We applied our recently proposed, dense dilated U-Net segmentation model, called ChoroidNET, for segmenting the choroid layer and choroidal vessels for our RP dataset. Choroid segmentation is an important task since the measurement results depend on it. Comparison with other state-of-the-art models shows that ChoroidNET provides a better quantitative and qualitative segmentation of the choroid layer and choroidal vessels. Next, we measure the choroidal parameters based on the segmentation results of ChoroidNET. The proposed method achieves high reliability with an intraclass correlation coefficient (0.961, 0.940, 0.826, 0.916) for TCA, LA, SA, and CT, respectively.







Similar content being viewed by others
References
Milam AH, Li ZY, Fariss RN (1998) Histopathology of the human retina in retinitis pigmentosa. Prog Retina Eye Res 17(2):175–205
Berson EL (1993) Retinitis pigmentosa: the Friedenwald lecture. Investig Ophthalmol Vis Sci 34(5):1659–1676
Koyanagi Y, Akiyama M, Nishiguchi KM et al (2019) Genetic characteristics of retinitis pigmentosa in 1204 Japanese patients. J Med Genet. https://doi.org/10.1136/jmedgenet-2018-105691
Konieczka K, Flammer AJ, Todorova M, Meyer P, Flammer J (2012) Retinitis pigmentosa and ocular blood flow. EPMA J 3(1):17
Egawa M, Mitamura Y, Niki M, Sano H, Miura G, Chiba A, Yamamoto S, Sonoda S, Sakamoto T (2018) Correlations between choroidal structures and visual functions in eyes with retinitis pigmentosa. Retina J Retinal Vitreous Dis 39(12):2399–2409
Cai CX, Locke KG, Ramachandran R, Birch DG, Hood DC (2014) A comparison of progressive loss of the ellipsoid zone (EZ) band in autosomal dominant and X-linked retinitis pigmentosa. Investig Ophthalmol Vis Sci 55:7417–7422
Chiba A, Miura G, Baba T, Yamamoto S (2019) Determination of length of interdigitation zone by optical coherence tomography and retinal sensitivity by microperimetry and their relationship to progression of retinitis pigmentosa. Biomed Res Int 2019:1217270
Son G, Lee S, Kim YJ, Lee JY, Kim J, Yoon YH (2019) Correlation between visual function and structural characteristics of the macula in advanced retinitis pigmentosa. Ophthalmologica 242(1):22–30
Ayton LN, Franzco RHG, Luu CD (2013) Choroidal thickness profiles in retinitis pigmentosa. Clin Exp Ophthalmol 41:396–403
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA, Fujimoto JG (1991) Optical coherence tomography. Science 254(5035):1178–1181
Jaffe GJ, Caprioli J (2004) Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol 137(1):156–169
Sezer T, Altinisik M, Koytak AK, Ozdemir MH (2016) The choroid and optical coherence tomography. Turk J Ophthalmol 46:30–37
Spaide RF, KoizumiPozonni HMC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 146(4):496–500
Orduna-Azcona J, Perez-Fernandez E, Guadilla AM, Manuel-Triantafilo SD, Modamio L, Gili P (2021) Sensitivity and specificity of choroidal thickness measurement by EDI-OCT for central serous chorioretinopathy diagnosis. Int Ophthalmol 41:257–264
Egawa M, Mitamura Y, Akaiwa K, Semba K, Kinoshita T, Uchino E, Sonoda S, Sakamoto T (2016) Changes of choroidal structure after corticosteroid treatment in eyes with Vogt–Koyanagi–Harada disease. Br J Ophthalmol 100:1646–1650
Hosein-Yazdi H, Vincent SJ, Collins MJ, Read SA, Alonso-Caneiro D (2019) Wide-field choroidal thickness in myopes and emmetropes. Sci Rep 9:3474. https://doi.org/10.1038/s41598-019-39653-w
Khaing TT, Okamoto T, Ye C, Mannan MA, Yokouchi H, Nakano K, Aimmanee P, Makhanov SS, Haneishi H (2021) ChoroidNET: a dense dilated U-Net model for choroid layer and vessel segmentation in optical coherence tomography images. IEEE Access 9:150951–150965. https://doi.org/10.1109/ACCESS.2021.3124993
Devalla SK, Renukanand PK, Sreedhar BK, Subramanian G, Zhang L, Perera S, Mari J, Chin KS, Tun TA, Strouthidis NG, Aung T, Thiery AH, Girard MJA (2018) DRUNET: a dilated-residual U-Net deep learning network to segment optic nerve head tissues in optical coherence tomography images. Biomed Opt Express 9(7):3244–3264
Zheng G, Jiang Y, Shi C, Miao H, Yu X, Wang Y, Chen S, Lin Z, Wang W, Lu F, Shen M (2021) Deep learning algorithms to segment and quantify the choroidal thickness and vasculature in swept-source optical coherence tomography images. J Innov Opt Health Sci 14(1):2140002
Rasband WS (1997) ImageJ, National Institutes of Health, Bethesda, Maryland, USA. https://imagej.nih.gov/ij/. Accessed 6 Nov 2021
Esmaeelpour M, Kajic V, Zabihian B et al (2014) Choroidal Haller’s and Sattler’s layer thickness measurement using 3-dimensional 1060-nm optical coherence tomography. PLoS ONE 9(6):e99690
Kajic V, Esmaeelpour M, Povazay B, Marshall D, Rosin PL, Drexler W (2012) Automated choroidal segmentation of 1060 nm OCT in healthy and pathologic eyes using a statistical model. Biomed Opt Express 3(1):86–103
Kajic V, Esmaeelpour M, Glittenberg C, Karus MF, Honegger J, Othara R, Binder S, Fujimoto JG, Drexler W (2013) Automated three-dimensional choroidal vessel segmentation of 3D 1060nm OCT retinal data. Biomed Opt Express 4(1):134–150
Wang E, Zhao X, Yang J, Chen Y (2020) Visualization of deep choroidal vasculatures and measurement of choroidal vascular density: a swept-source optical coherence tomography angiography approach. BMC Ophthalmol 20:321. https://doi.org/10.1186/s12886-020-01591-x
Shen C, Li Y, Wang Q, Chen Y, Li W, Wei W (2020) Choroidal vascular changes in retinitis pigmentosa patients detected by optical coherence tomography angiography. BMC Ophthalmol 20:384. https://doi.org/10.1186/s12886-020-01640-5
Ronneberger O, Fischer P, Brox T (2015) U-net: convolutional networks for biomedical image segmentation. In: International conference on medical image computing and computer-assisted intervention (MICCAI), pp 234–241
Kanopoulos N, Vasanthavada N, Baker RL (1988) Design of an image edge detection filter using the Sobel operator. IEEE J Solid State Circuits 23(2):358–367
Zhou Z, Siddiquee MMR, Tajbakhsh N, Liang J (2018) U-Net++: a nested architecture for medical image segmentation. In: Computer vision and pattern recognition. arXiv:1807.10165[cs.CV]
Stralen KJV, Jager KJ, Zoccali C, Dekker FW (2008) Agreement between methods. Kidney Int 74:1116–1120
Liu G, Liu X, Li H, DuWang QF (2016) Optical coherence tomography analysis of retina in retinitis pigmentosa patients. Ophthalmic Res 56:111–122
Acknowledgements
The authors would like to acknowledge the financial support provided by the KAKENHI under the Grant-in-Aid for Scientific Research (A) (Grant number 19H01172), the Japan Society for the Promotion of Science (JSPS) Core-to-Core Program (JPJSCCA20170004), the Thai Government Research Fund (contract numbers 33/2560 and 24/2561), the National Research Council of Thailand (NRCT) (Grant number NRCT5-RSA63010-05), and the Center of Excellence in Biomedical Engineering of Thammasat University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Khaing, T.T., Okamoto, T., Ye, C. et al. Automatic measurement of choroidal thickness and vasculature in optical coherence tomography images of eyes with retinitis pigmentosa. Artif Life Robotics 27, 70–79 (2022). https://doi.org/10.1007/s10015-022-00737-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10015-022-00737-y