Abstract
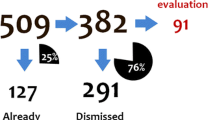
This paper introduces a method for mining co-occurring events from longitudinal data, and applies this method to detecting adverse drug reactions (ADRs) from patient data. Electronic health records are richer than older data sources (such as spontaneous report records) and thus are ideal for ADR mining. However, current data mining methods, such as disproportionality ratios and temporal itemset mining, ignore certain important aspects of the longitudinal data in patient records. In this paper, we highlight two specific problems with current methods, which we name temporal and contextual sensitivity, and discuss why these two properties are vital to mining patterns from longitudinal data. We also propose two sensitive longitudinal rate comparison measures, which utilize condition occurrence rates and length of drug eras, for mining ADRs from this type of data. These novel methods are then used to rank potential ADRs, along with existing state-of-the-art methods, under many simulated yet realistic datasets. In 48 out of 60 experiments, the proposed longitudinal rate comparison methods significantly outperform other methods in mining known ADRs from other drug / condition pairs.




Similar content being viewed by others
References
Agrawal R, Imielinski T, Swami A. Mining association rules between sets of items in large databases. In: Proceedings of the 1993 ACM SIGMOD International Conference on Management of Data, SIGMOD ’93, pp. 207–216. ACM, New York, NY, US (1993). doi:10.1145/170035.170072
Ahmed I, Dalmasso C, Haramburu F, Thiessard F, Brot P, Tubert-Bitter P (2010) False discovery rate estimation for frequentist pharmacovigilance signal detection methods. Biom 66(1):301–309. doi:10.1111/j.1541-0420.2009.01262.x
Bate A (2003) The use of bayesian confidence propagation neural network in pharmacovigilance. Ph.D. thesis. Ume University, Pharmacology and Clinical Neuroscience
Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, De Freitas RM (1998) A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol 54(4):315—321
DS B, DA P, KN W, AB M, TJ S, JL A (2006) JAMA 296(15):1858–1866. doi:10.1001/jama.296.15.1858
Dumouchel W (1999) Bayesian data mining in large frequency tables, with an application to the fda spontaneous reporting system. Am Stat 53(3):177–190. doi:10.1080/00031305.1999.10474456
DuMouchel W, Pregibon D Empirical bayes screening for multi-item associations. Proceedings of the seventh ACM SIGKDD international conference on Knowledge discovery and data mining pp. 67–76 (2001). doi:10.1145/502512.502526
Ernst FR, Grizzle AJ (2001) Drug-related morbidity and mortality: updating the cost-of-illness model. J Am Pharm Assoc (Wash) 41(2):192–199
Evans S (2002) Statistical methods of signal detection. John Wiley & Sons, pp 273–279. Ltd. doi:10.1002/0470853093.ch20
Evans SJ, Waller PC, Davis S (2001) Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf 10(6):483–486. doi:10.1002/pds.677
Hauben M, Reich L (2005) Potential utility of data-mining algorithms for early detection of potentially fatal/disabling adverse drug reactions: a retrospective evaluation. J Clin Pharmacol 45(4):378–384
Krishnamoorthy K, Thomson J (2004) A more powerful test for comparing two poisson means. J Stat Plan Infer 119(1):23–35
Li Y, Ning P, Wang X, Jajodia S (2003) Discovering calendar-based temporal association rules. Data Knowl Eng 44(2):193–218. http://www.sciencedirect.com/science/article/pii/S0169023X02001350
Lin S, Qiao J, Wang Y (2014) Frequent episode mining within the latest time windows over event streams. Appl Intell 40(1):13–28. doi:10.1007/s10489-013-0442-8
Lindquist M, Stahl M, Bate A, Edwards IR, Meyboom R.H.B. (2000) A retrospective evaluation of a data mining approach to aid finding new adverse drug reaction signals in the WHO international database. Drug Saf 23(6):533– 542
Liu W, Zheng Y, Chawla S, Yuan J, Xing X Discovering spatio-temporal causal interactions in traffic data streams. In: Proceedings of the 17th ACM SIGKDD International Conference on Knowledge Discovery and Data Mining, KDD ’11, pp. 1010–1018. ACM, New York, NY, USA (2011). doi:10.1145/2020408.2020571
Mannila H, Toivonen H Discovering generalized episodes using minimal occurrences. In KDD 96: Proc. 2nd International Conference on Knowledge Discovery and Data Mining, pp. 146–151. AAAI Press (1996)
Mohan P, Shekhar S, Shine J, Rogers J (2012) Cascading spatio-temporal pattern discovery. Knowl Data Eng IEEE Trans 24(11):1977–1992. doi:10.1109/TKDE.2011.146
Moore N, Kreft-Jais C, Haramburu F, Noblet C, Andrejak M, Ollagnier M, Bgaud B (1997) Reports of hypoglycaemia associated with the use of ACE inhibitors and other drugs: a case/non-case study in the French pharmacovigilance system database. Br J Clin Pharmacol 44(5):513–518
Murray RE, Ryan PB, Reisinger SJ (2011) Design and validation of a data simulation model for longitudinal healthcare data. AMIA Annu Symp Proc 2011:1176–1185
Noren G, Hopstadius J, Bate A, Star K, Edwards I (2010) Temporal pattern discovery in longitudinal electronic patient records. Data Min Knowl Discov 20(3):361–387. doi:10.1007/s10618-009-0152-3
(OMOP) OMOP Observational medical dataset simulator generation 1 (2009). Available from OMOP at http://www.omop.org
Przyborowski J, Wilenski H (1940) Homogeneity of results in testing samples from poisson series: With an application to testing clover seed for dodder. Biometrika 31(3-4):313–323. http://biomet.oxfordjournals.org/content/31/3-4/313.short
Sakaeda T, Tamon A, Kadoyama K, Okuno Y (2013) Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci 10(7):796–803
Food U.S., Administration Drug FAERS Patient Outcomes by Year. U.S. Food and Drug Administration (2012). http://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/surveillance
Yen SJ, Lee YS (2013) Mining non-redundant time-gap sequential patterns. Appl Intell 39(4):727–738. doi:10.1007/s10489-013-0426-8
Zorych I, Madigan D, Ryan P, Bate A (2013) Disproportionality methods for pharmacovigilance in longitudinal observational databases. Stat Methods Med Res 22(1):39–56
Acknowledgments
The authors would like to thank MITRE corporation for its support of this project in 2013.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lo, H., Ding, W. & Nazeri, Z. Temporality and Context for detecting adverse drug reactions from longitudinal data. Appl Intell 41, 1069–1080 (2014). https://doi.org/10.1007/s10489-014-0568-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10489-014-0568-3