Abstract
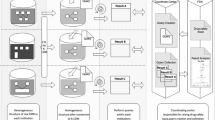
An adverse drug reaction (ADR) surveillance system integrated with various electronic medical record (EMR) systems has been suggested as an effective way to collect more data and analyze ADRs earlier than the spontaneous reporting of ADRs. Because Korean hospitals have heterogeneous EMR databases, a common data model (CDM) should first be defined to develop the multi-center EMR-based drug surveillance system. We investigated the data models from two prominent drug safety surveillance studies, the Mini-Sentinel program and the Observational Medical Outcomes Partnership, and developed an EMR-based ADR common data model (EADR CDM). The EADR CDM has eight tables, including a demographic table, drug table, visit table, procedure table, diagnosis table, death table, laboratory table and organization table. Each table consists of 5–12 fields. Among a total of 2,931,060 patients from January 2008 to December 2012 in clinical data warehouse of the S hospital, we extracted the data from 135,745 patients who were prescribed below drugs to determine whether the exported data were sufficient to detect ADRs of six drugs. After validation, we found that the transformed data based on EADR CDM is helpful to understand the prescription pattern and explore feasible medication list for adverse drug signal detection. The collection of diverse data using the CDM is an effective method of early decision of ADRs. This study provides guidelines for developing the CDM and plans to develop the drug safety surveillance system based on multi-center EMR.


Similar content being viewed by others
References
World Health Organization (2002) The importance of pharmacovigilance? Safety monitoring of medicinal products, World Health Organization, Geneva. http://apps.who.int/medicinedocs/pdf/s4893e/s4893e.pdf. Accessed 6 Mar 2013
Rodriguez EM, Staffa JA, Graham DJ (2001) The role of databases in drug postmarketing surveillance. Pharmacoepidemiol Drug Saf 10(5):407–410
Jha AK, Kuperman GJ, Teich JM, Leape L, Shea B, Eve R, Burdick E, Seger DL, Vliet MV, Bates DW (1998) Identifying adverse drug events development of a computer-based monitor and comparison with chart review and stimulated voluntary report. J Am Med Inform Assoc 5:305–314
Platt R, Carnahan RM, Brown JS, Chrischilles E, Curtis LH, Hennessy S, Nelson JC, Racoosin JA, Robb M, Schneeweiss S, Toh S, Weiner MG (2012) The U.S. food and drug administration’s mini-sentinel program: status and direction. Pharmacoepidemiol Drug Saf 21:1–8
Curtis LH, Weiner MG, Boudreau DM, Cooper WO, Daniel GW, Nair VP, Raebel MA, Beaulieu NU, Rosofsky R, Woodworth TS, Brown JS (2012) Design considerations, architecture, and use of the mini-sentinel distributed data system. Pharmacoepidemiol Drug Saf 21:23–31
Forrow S, Campion DM, Herrinton LJ, Nair VP, Robb MA, Wilson M, Platt R (2012) The organizational structure and governing principles of the Food and Drug Administration’s Mini-Sentinel pilot program. Pharmacoepidemiol Drug Saf 21:12–17
Stang PE, Ryan PB, Racoosin JA, Overhage JM, Hartzema AG, Reich C, Welebob E, Scarneccia T, Woodcock J (2010) Advancing the science for active surveillance: rationale and design for the Observational Medical Outcomes Partnership. Ann Intern Med 153:600–606
Trifiro G, Fourrier-Reglat A, Sturkenboom MC, Díaz Acedo C, Lei JVD (2009) The EU-ADR project: preliminary results and perspective. Stud Health Technol Inform 148:43–49
Coloma PM, Schuemie MJ, Trifirò G, Gini R, Herings R, Hippisley-Cox J, Mazzaglia G, Giaquinto C, Corrao G, Pedersen L, Van Der Lei J, Sturkenboom M (2011) Combining electronic healthcare databases in Europe to allow for large-scale drug safety monitoring: the EU-ADR Project. Pharmacoepidemiol Drug Saf 20:1–11
Hwang SH, Kim EY, Lee YS, Jung SY, Lee YM, Son KH, Choi KU, Lee SH, Kim Y (2005) Implementation and evaluation of the computerized surveillance system to identify adverse drug events: pilot study. J Korean Soc Health Syst Pharm 22(2):118–136
Lee YH, Yoon YM, Lee BM, Hwang HJ, Kang UG (2009) Development of mining model through reproducibility assessment in adverse drug event surveillance system. Korean Soc Comput Inf 1(3):183–192
Lee YH, Kang UG, Park RW (2009) Development of adverse drug event surveillance system using BI Technology. J Korean Contents 9(2):106–114
Le HV, Beach KJ, Powell G, Pattishall ED, Ryan P, Mera RM (2013) Performance of a semi-automated approach for risk estimation using a common data model for longitudinal healthcare databases. Stat Methods Med Res 22(1):97–112
Kim HS, Cho H, Lee IK (2011) Design and development of an EHR platform based on medical informatics standards. J Fuzzy Log Intell Syst 21:456–462
Cook AJ, Tiwari RC, Wellman RD, Heckbert SR, Li L, Heagerty P, Marsh T, Nelson JC (2012) Statistical approaches to group sequential monitoring of postmarket safety surveillance data: current state of the art for use in the Mini-Sentinel pilot. Pharmacoepidemiol Drug Saf 21:72–81
Stang PE, Ryan PB, Dusetzina SB, Hartzema AG, Reich C, Overhage JM, Racoosin JA (2012) Health outcomes of interest in observational data: issues in identifying definitions in the literature. Health Outcomes Res Med 3(1):e37–e44
Murphy SN, Castro V, Colecchi J, Dubey A, Gainer V, Herrick C, Sordo M (2011) Partners HealthCare OMOP Study Report. Foundation for the National Institutes of Health Observational Medical Outcomes Partnership Partners HealthCare Jan 10
Overhang JM, Ryan PB, Reich CG, Hartzema AG, Stang PE (2012) Validation of a common data model for active safety surveillance research. Am Med Inform Assoc 19:54–60
Sung ES, Kim TH, Cho SH, Kim KR, Park CW, Jeong JH (2012) Epistaxis in patients taking oral anticoagulant and antiplatelet medication. Korean J Otorhinolaryngol Head Neck Surg 55(5):290–294. doi:10.3342/kjorl-hns.2012.55.5.290
Lee MW, Lee JS, Han OY, Choi IY, Jeong SH, Yim HW, Lee DG, La HO, Park YM (2014) Study for association between adverse drug reactions and causative drugs in the elderly using data-mining analysis. Korean J Clin Pharm 24(1):39–44
Acknowledgments
This study was supported by a grant of the Korea Health technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A112022).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rho, M.J., Kim, S.R., Park, S.H. et al. Common data model for decision support system of adverse drug reaction to extract knowledge from multi-center database. Inf Technol Manag 17, 57–66 (2016). https://doi.org/10.1007/s10799-015-0240-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10799-015-0240-6