Summary
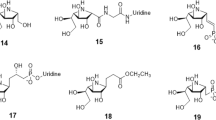
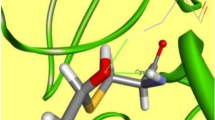
For AIDS therapy, there are currently a number of compounds available for multiple targets already approved by the FDA and in clinic, e.g. protease inhibitors, reverse transcriptase inhibitors (NRTI, NNRTI), fusion inhibitors, CCR4, CCR5 among others. Some pharmaceuticals act against the virus before the entrance of HIV into the host cells. One of these targets is the glucosidase protein. This novel fusion target has been recently explored because the synthesis of viral glycoproteins depends on the activity of enzymes, such as glucosidase and transferase, for the elaboration of the polysaccharides. In this work we have built an homology model of Saccharomyces cerevisiae glucosidase and superimposed all relevant glucosidase-like enzymes in complex with carbohydrates, and calculated as well molecular interaction fields in our S. cerevisiae active site model. Our results suggest that there are two saccharide binding sites which are the most important for the binding of inhibitors with this family of enzymes which supports the possibility of inhibitors containing only two sugar units. Based on these results, we have proposed a novel pseudo-dissacharide which is a potential pharmaceutical for AIDS treatment.
Similar content being viewed by others
References
J.B.L. Martins C.A. Taft M.A. Perez F.M.L.G. Stamato E. Longo (1998) Int. J. Quant. Chem. 69 117 Occurrence Handle10.1002/(SICI)1097-461X(1998)69:1<117::AID-QUA13>3.0.CO;2-3 Occurrence Handle1:CAS:528:DyaK1cXktVekt7s%3D
Pavão, A.C., Taft, C.A., Guimarãles, T.C.F., Leão, M.B.C., Mohallem, J.R., Lester, W.A.Jr., J. Phys. Chem. A, 105 (2001) 5 – Invited International Paper: Special edition dedicated to Per-Olov LÖwdin
J.B.L. Martins M.A. Perez E. Longo C.A. Taft M. Arissawa F.G.L. Stamato J.G.R. Tostes (2002) Int. J. Quant. Chem. 90 575 Occurrence Handle10.1002/qua.987 Occurrence Handle1:CAS:528:DC%2BD38XntlOitrg%3D
X. Fradera X. de la Cruz C.H.T.P. Silva J.L. Gelpi F.S. Luque M. Orozco (2002) Bioinformatics 18 939 Occurrence Handle10.1093/bioinformatics/18.7.939 Occurrence Handle1:CAS:528:DC%2BD38XmtVaqt7o%3D
M. Arissawa C.A. Taft J. Felcman C.H.T. Silva C.A. Taft (2003) Int. J. Quant. Chem. 93 422 Occurrence Handle10.1002/qua.10580 Occurrence Handle1:CAS:528:DC%2BD3sXksFSiurw%3D
C.H.T.P. Silva P. Almeida C.A. Taft (2004) J. Mol. Model. 10 38 Occurrence Handle10.1007/s00894-003-0167-4 Occurrence Handle1:CAS:528:DC%2BD2cXktFSgtLw%3D
C.H.T.P. Silva S.M. Sanches C.A. Taft (2004) J. Mol. Graph. Model. 23 89 Occurrence Handle10.1016/j.jmgm.2004.03.013 Occurrence Handle15331057
I. Carvalho E.B. Melo (2004) Carbohyd. Res. 339 361 Occurrence Handle10.1016/j.carres.2003.10.010 Occurrence Handle1:CAS:528:DC%2BD3sXhtVWhu7jP
P.R. Walker M. Worobey A. Rambaut E.C. Holmes O.G. Pybus (2003) Nature 422 6933 Occurrence Handle10.1038/422679a
M.A. Noor R.A. Parker E. O’Mara D.M. Grasela A. Currie S.L. Hodder F.T. Fiedorek D.W. Haas (2004) AIDS 18 2137 Occurrence Handle10.1097/00002030-200411050-00005 Occurrence Handle1:CAS:528:DC%2BD2cXhtVOqt7jK Occurrence Handle15577646
T.M. Dando L.J. Scott (2005) Drugs 65 285 Occurrence Handle15631548
A.S. Veiga N.C. Santos L.M. Loura A. Fedorov M.A. Castanho (2004) J. Am. Chem. Soc. 126 14758 Occurrence Handle10.1021/ja0459882 Occurrence Handle1:CAS:528:DC%2BD2cXovFSlsrw%3D Occurrence Handle15535700
K. Princen S. Hatse K. Vermeire S. Aquaro E. De Clercq L.O. Gerlach M. Rosenkilde T.W. Schwartz R. Skerlj G. Bridger D. Schols (2004) J. Virol. 78 12996 Occurrence Handle10.1128/JVI.78.23.12996-13006.2004 Occurrence Handle1:CAS:528:DC%2BD2cXhtVaqtbnP Occurrence Handle15542651
A. Mehta N. Zitzmann P.M. Rudd T.M. Block R.A. Dwek (1998) FEBS Lett. 430 17 Occurrence Handle10.1016/S0014-5793(98)00525-0 Occurrence Handle1:CAS:528:DyaK1cXktFGmur4%3D Occurrence Handle9678587
N. Asano M. Nishida A. Kato H. Kizu K. Matsui Y. Shimada T Itoh M. Baba A.A. Watson R.J. Nash P.M. Lilley Particlede D.J. Watkin G.W.J. Fleet (1998) J. Med. Chem. 41 2565 Occurrence Handle10.1021/jm970836l Occurrence Handle1:CAS:528:DyaK1cXkt12jt7Y%3D Occurrence Handle9651160
D.C. Billington F. Perron-Sierra I. Picard S. Beaubras J. Duhault J. Espinal S. Challal (1994) Bioorg. Med. Chem. Lett. 19 2307 Occurrence Handle10.1016/0960-894X(94)85030-5
P.I. Dalko P. Sinay (1999) Angew. Chem. Int. Edit. 38 773 Occurrence Handle10.1002/(SICI)1521-3773(19990315)38:6<773::AID-ANIE773>3.0.CO;2-N Occurrence Handle1:CAS:528:DyaK1MXisVelu7Y%3D
A. Sali T.L. Blundell (1990) J. Mol. Biol. 212 403 Occurrence Handle10.1016/0022-2836(90)90134-8 Occurrence Handle1:CAS:528:DyaK3cXitlWmsbc%3D Occurrence Handle2181150
A. Roujeinikova C. Raasch S. Sedelnikova W. Liebl D.W. Rice (2002) J. Mol. Biol. 321 149 Occurrence Handle10.1016/S0022-2836(02)00570-3 Occurrence Handle1:CAS:528:DC%2BD38XlsFKgtbY%3D Occurrence Handle12139940
G. Barton M.J.E. Sternberg (1987) J. Mol. Biol. 198 327 Occurrence Handle10.1016/0022-2836(87)90316-0 Occurrence Handle1:CAS:528:DyaL1cXlt1ersA%3D%3D Occurrence Handle3430611
InstitutionalAuthorNameInsight II. (2004) Accelrys CA San Diego
J.L. Gelpi S.G. Kalko X. Barril J. Cirera X. Cruz Particlede la F.J. Luque M. Orozco (1991) Proteins 45 110
M. Blanco (1991) J. Comp. Chem. 12 237 Occurrence Handle10.1002/jcc.540120214 Occurrence Handle1:CAS:528:DyaK3MXhsFSns7s%3D
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, C., Carvalho, I. & Taft, C. Homology modeling and molecular interaction field studies of α-glucosidases as a guide to structure-based design of novel proposed anti-HIV inhibitors. J Comput Aided Mol Des 19, 83–92 (2005). https://doi.org/10.1007/s10822-005-1486-6
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10822-005-1486-6