Abstract
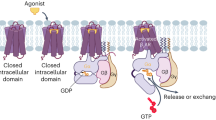
Adrenoceptors are members of the important G protein coupled receptor family for which the detailed mechanism of activation remains unclear. In this study, we have combined docking and molecular dynamics simulations to model the ligand induced effect on an homology derived human α1A adrenoceptor. Analysis of agonist/α1A adrenoceptor complex interactions focused on the role of the charged amine group, the aromatic ring, the N-methyl group of adrenaline, the beta hydroxyl group and the catechol meta and para hydroxyl groups of the catecholamines. The most critical interactions for the binding of the agonists are consistent with many earlier reports and our study suggests new residues possibly involved in the agonist-binding site, namely Thr-174 and Cys-176. We further observe a number of structural changes that occur upon agonist binding including a movement of TM-V away from TM-III and a change in the interactions of Asp-123 of the conserved DRY motif. This may cause Arg-124 to move out of the TM helical bundle and change the orientation of residues in IC-II and IC-III, allowing for increased affinity of coupling to the G-protein.
Similar content being viewed by others
References
M.C. Beduschi R. Beduschi J.E. Oesterling (1998) Urol. 51 861 Occurrence Handle10.1016/S0090-4295(98)00140-X Occurrence Handle9609620
J. Ballesteros K. Palczewski (2001) Curr. Opin. Drug Disc. Devel. 4 561
T. Okada K. Palczewski (2001) Curr. Opin. Struct. Biol. 11 420 Occurrence Handle10.1016/S0959-440X(00)00227-X Occurrence Handle11495733
G.K. Kinsella I. Rozas G.W. Watson (2004) Biochem. Biophys. Res. Commun. 324 916 Occurrence Handle10.1016/j.bbrc.2004.09.128 Occurrence Handle15474515
Hamaguchi, N., True, T., Saussy, D.L., Jeffs P.W., Biochemistry, 35 (1996) 14312.
M. Zhao J. Hwa D.M. Perez (1996) Mol. Pharmacol. 50 1118 Occurrence Handle8913343
D. Waugh R. Gaivin M. Zuscik P. Gonzalez-Cabrera S. Ross J. Yun D. Perez (2001) J. Biol. Chem. 27 25366 Occurrence Handle10.1074/jbc.M103152200
A. Cavalli F. Fanelli C. Taddei P.G. Benedetti Particlede S. Cotechia (1996) FEBS Lett. 399 9 Occurrence Handle10.1016/S0014-5793(96)01286-0 Occurrence Handle8980109
M.T. Piascik D.M. Perez (2001) J. Pharmacol. Exp. Ther. 298 2
B. Kobilka (2004) Mol. Pharmacol. 65 1060 Occurrence Handle10.1124/mol.65.5.1060 Occurrence Handle15102933
U. Gether B.K. Kobilka (1998) J. Biol. Chem. 273 IssueID29 17979 Occurrence Handle10.1074/jbc.273.29.17979 Occurrence Handle9660746
J.A. Javitch D. Fu G. Liapakis J. Chen (1997) J. Biol. Chem. 272 18546 Occurrence Handle10.1074/jbc.272.30.18546 Occurrence Handle9228019
I. Bea C. Jaime P. Kollman (2002) Theor. Chem. Acta. 108 286
C. Bissantz P. Bernard M. Hibert D. Rognan (2003) Proteins 50 5 Occurrence Handle10.1002/prot.10237 Occurrence Handle12471595
J.J. Chambers D.E. Nichols (2002) J. Comput. Aided Mol. Des. 16 511 Occurrence Handle10.1023/A:1021275430021 Occurrence Handle12510883
R. Carmine P. Molinari M. Sbraccia C. Ambrosio T. Costa (2004) Mol. Pharmacol. 66 2
I.D. Kuntz (1992) Science 257 1078 Occurrence Handle1509259
Sybyl6.9, Molecular Modelling System Tripos Associates. St. Louis, MO, USA.
M.J. Frisch G.W. Trucks H.B. Schlegel G.E. Scuseria M.A. Robb J.R. Cheeseman V.G. Zakrzewski J.A. Montgomery SuffixJr. R.E. Stratmann J.C. Burant S. Dapprich J.M. Millam A.D. Daniels K.N. Kudin M.C. Strain O. Farkas J. Tomasi V. Barone M. Cossi R. Cammi B. Mennucci C. Pomelli C. Adamo S. Clifford J. Ochterski G.A. Petersson P.Y. Ayala Q. Cui K. Morokuma P. Salvador J.J. Dannenberg D.K. Malick A.D. Rabuck K. Raghavachari J.B. Foresman J. Cioslowski J.V. Ortiz A.G. Baboul B.B. Stefanov G. Liu A. Liashenko P. Piskorz I. Komaromi R. Gomperts R.L. Martin D.J. Fox T. Keith M.A. Al-Laham C.Y. Peng A. Nanayakkara M. Challacombe P.M.W. Gill B. Johnson W. Chen M.W. Wong J.L. Andres C. Gonzalez M. Head-Gordon E.S. Replogle J.A. Pople (2001) Gaussian 98 (Rev A.11) Gaussian, Inc Pittsburgh, PA
D.A. Case D.A. Pearlman J.W. Caldwell T.E. Cheatham SuffixIII. J. Wang W.S. Ross C.L. Simmerling T.A. Darden K.M. Merz R.V. Stanton A.L. Cheng J.J. Vincent M. Crowley V. Tsui H. Gohlke R.J. Radmer Y. Duan J. Pitera I. Massova G.L. Seibel U.C. Singh P.K. Weiner P.A. Kollman (2001) Amber 7 University of California San Francisco
J. Wang P. Cieplak P.A. Kollman (2000) J. Comput. Chem. 21 1049 Occurrence Handle10.1002/1096-987X(200009)21:12<1049::AID-JCC3>3.0.CO;2-F
H.J.C. Berendsen J.P.M. Postma W.F. Gunsteren Particlevan A. DiNola J.R. Haak (1984) J. Chem. Phys. 81 3684 Occurrence Handle10.1063/1.448118
Wymore, T. and Wong, T.C., 45th Annual Meeting of the Biophysical Society. Boston, MA, 2001.
J. Wang R.M. Wolf W. James J.W. Caldwell P.A. Kollman D.A. Case (2004) J. Comp. Chem. 25 1157 Occurrence Handle10.1002/jcc.20035
Aldrich Library of FT-IR Spectra, 1 (1) 1296A.
A. Pedretti M.E. Silva L. Villa G. Vistoli (2000) Biochem. Biophys. Res. Commun. 319 493 Occurrence Handle10.1016/j.bbrc.2004.04.149
I. Visiers J.A. Ballesteros H. Weinstein (2002) Methods Enzymol. 343 329 Occurrence Handle11665578
K. Palczewski T. Kumasaka T. Hori C.A. Behnke H. Motoshima B.A. Fox I.L. Trong D.C. Teller T. Okada R.E. Stenkamp M. Yamamoto M. Miyano (2000) Science 289 739 Occurrence Handle10.1126/science.289.5480.739 Occurrence Handle10926528
X. Luo D. Zhang H. Weinstein (1994) Protein Eng. 7 1441 Occurrence Handle7716154
A. Scheer F. Fanelli T. Costa P.G. Benedetti ParticleDe S. Cotecchia (1996) Embo J. 15 3566 Occurrence Handle8670860
D. Zhang H. Weinstein (1993) J. Med. Chem. 36 934 Occurrence Handle10.1021/jm00059a021 Occurrence Handle8464048
D.L. Farrens C. Altenbach K. Yang W.L. Hubbell H.G. Khorana (1996) Science 274 768 Occurrence Handle10.1126/science.274.5288.768 Occurrence Handle8864113
U. Gether J.A. Ballesteros R. Seifert E. Sanders-Bush H. Weinstein B.K. Kobilka (1997) J. Biol. Chem. 272 2587 Occurrence Handle10.1074/jbc.272.5.2587 Occurrence Handle9006889
U. Gether S. Lin P. Ghanouni J.A. Ballesteros H. Weinstein B.K. Kobilka (1997) Embo J. 16 6737 Occurrence Handle10.1093/emboj/16.22.6737 Occurrence Handle9362488
U. Gether S. Lin B.K. Kobilka (1995) J. Biol. Chem. 270 28268 Occurrence Handle10.1074/jbc.270.47.28268 Occurrence Handle7499324
J.A. Javitch D. Fu G. Liapakis J. Chen (1997) J. Biol. Chem. 272 18546 Occurrence Handle10.1074/jbc.272.30.18546 Occurrence Handle9228019
C.D. Strader T.M. Fong M.R. Tota D. Underwood R.A. Dixon (1994) Annu Rev. Biochem. 63 101 Occurrence Handle10.1146/annurev.bi.63.070194.000533 Occurrence Handle7979235
J. Wess (1998) Pharmacol. Therap. 80 231 Occurrence Handle10.1016/S0163-7258(98)00030-8
W. Zhou C. Flanagan J.A. Ballesteros K. Konvocka J.S. Davidson H. Weinstein R.P. Millar S.C. Sealfon (1994) Mol. Pharmacol. 45 165 Occurrence Handle8114667
S.C. Sealfon L. Chi B.J. Ebersole V. Rodic D. Zhang J.A. Ballesteros H. Weinstein (1995) J. Biol. Chem. 270 16683 Occurrence Handle10.1074/jbc.270.28.16683 Occurrence Handle7622478
Jongejan A., Bruysters M., Pardo L., Leurs R., Oral Presentation XVII Symposium of Medicinal Chemistry, 2004.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kinsella, G.K., Rozas, I. & Watson, G.W. Modelling the Interaction of Catecholamines with the α1A Adrenoceptor Towards a Ligand-induced Receptor Structure. J Comput Aided Mol Des 19, 357–367 (2005). https://doi.org/10.1007/s10822-005-7553-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10822-005-7553-1