Abstract
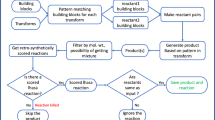
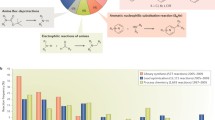
AllChem is a system that is intended to make practical the generation and searching of an unprecedentedly vast number (∼1020) of synthetically accessible and medicinally relevant structures. Also, by providing possible synthetic routes to a structure along with its design rationale, AllChem encourages simultaneous consideration of both costs and benefits during each lead discovery and optimization decision, thereby promising to be effective with synthetic chemists among its primary users. AllChem is still under intensive development so the following initial description necessarily has more the character of an interim progress report than of a finished research publication.







Similar content being viewed by others
References
Cramer RD, Soltanshahi F, Jilek R, Campbell B (2006) Abs ACS, 231st mtg: CINF 38
Todd MH (2005) Chem Soc Rev 34:247–266
Corey EJ, Wipke WT (1969) Science 166:178
Blurock ES (1990) J Chem Inf Comput Sci 30:505–510
Gelernter H, Rose JR, Chen C (1990) J Chem Inf Comput Sci 30:492–504
Helson HE, Jorgensen WL (1994) J Org Chem 59:3841–3856
Zeigarnik AV, Bruk LG, Temin ON, Likholobov VA, Maier LI (1996) Russ Chem Rev 65:117–130
Hendrickson JB (1997) Knowl Eng Rev 12:369–386
Mehta G, Barone R, Chanon M (1998) Eur J Org Chem 7:1409–1412
Matyska L, Koca J (1991) J Chem Inf Comput Sci 31:380–386
Young DC (2002) in Comp. Chem. Wiley, NYC, NY
Lushnikov DE, Zefirov NS (1992) J Chem Inf Comput Sci 32:317–322
Johnson AP, Marshall C, Judson PN (1992) J Chem Inf Comput Sci 32:411–417
Bertz SH, Rucker C, Rucker G, Sommer TJ (2003) Eur J Org Chem 24:4737–4740
Satoh H, Funatsu K (1995) J Chem Inf Comput Sci 35:34–44
Moll H (1994) J Chem Inf Comput Sci 34:117–119
Hanessian S, Franco J, Gagnon G, Laramee D, LaRouche B (1990) J Chem Inf Comput Sci 30:413–425
Ihlenfeldt W-D, Gasteiger J (1995) Angew Chem Intl 34:2613–2633
Caflisch A, Karplus M (1995) Pers Drug Disc Des 3:51–84
Jauffret P, Ostermann C, Kaufmann G (2003) Eur J Org Chem 10:1983–1992
Baber JC, Feher M (2004) Mini Rev Med Chem 4:681–692
Ugi I, Bauer J, Bley K, Dengler A, Dietz A, Fontain E, Gruber B, Herges R, Knauer M, Reitsam K, Stein N (1993) Angew Chem Intl Ed 32:201–227
Jilek RJ, Cramer RD (2004) J Chem Inf Comp Sci 44:1221–1227
Cramer RD, Jilek RJ, Guessregen S, Clark SJ, Wendt B, Clark RD (2004) J Med Chem 47:6777–6791
Cramer RD, Patterson DE, Clark RD, Soltanshahi F, Lawless MS (1998) J Chem Inf Comp Sci 6:1010–1023
Andrews KM, Cramer RD (2000) J Med Chem 43:1723–1740
Corey EJ, Cramer RD, Howe WJ (1972) J Am Chem Soc 94:440–459
Ertl P, Jelfs S, Muhlbacher J, Schuffenhauer A, Selzer P (2006) J Med Chem 49:4568–4573
Greene TW, Wuts PGM (1999) Protective groups in organic synthesis. Wiley, NYC, NY
Ash S, Cline MA, Homer RW, Hurst T, Smith GB (1997) J Chem Inf Comput Sci 37:71–79
Cramer RD, Clark RD, Patterson DE, Ferguson AM (1996) J Med Chem 39:3060–3069
Cramer RD (2003) J Med Chem 46:374–389
Acknowledgment
We are grateful to NIH/GMS for financial support of this development project, currently from award: SBIR 2 R44 GM068359-02.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cramer, R.D., Soltanshahi, F., Jilek, R. et al. AllChem: generating and searching 1020 synthetically accessible structures. J Comput Aided Mol Des 21, 341–350 (2007). https://doi.org/10.1007/s10822-006-9093-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-006-9093-8