Abstract
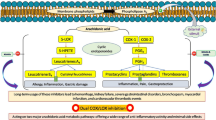
The human secretory phospholipase A2 group IIA (PLA2-IIA) is a lipolytic enzyme. Its inhibition leads to a decrease in eicosanoids levels and, thereby, to reduced inflammation. Therefore, PLA2-IIA is of high pharmacological interest in treatment of chronic diseases such as asthma and rheumatoid arthritis. Quercetin and naringenin, amongst other flavonoids, are known for their anti-inflammatory activity by modulation of enzymes of the arachidonic acid cascade. However, the mechanism by which flavonoids inhibit Phospholipase A2 (PLA2) remained unclear so far. Flavonoids are widely produced in plant tissues and, thereby, suitable targets for pharmaceutical extractions and chemical syntheses. Our work focuses on understanding the binding modes of flavonoids to PLA2, their inhibition mechanism and the rationale to modify them to obtain potent and specific inhibitors. Our computational and experimental studies focused on a set of 24 compounds including natural flavonoids and naringenin-based derivatives. Experimental results on PLA2-inhibition showed good inhibitory activity for quercetin, kaempferol, and galangin, but relatively poor for naringenin. Several naringenin derivatives were synthesized and tested for affinity and inhibitory activity improvement. 6-(1,1-dimethylallyl)naringenin revealed comparable PLA2 inhibition to quercetin-like compounds. We characterized the binding mode of these compounds and the determinants for their affinity, selectivity, and inhibitory potency. Based on our results, we suggest C(6) as the most promising position of the flavonoid scaffold to introduce chemical modifications to improve affinity, selectivity, and inhibition of PLA2-IIA by flavonoids.







Similar content being viewed by others
Abbreviations
- PLA2:
-
Phospholipase A2
- PLA2-IB:
-
Phospholipase A2 group IB
- PLA2-IIA:
-
Phospholipase A2 group IIA
- 3D:
-
Three-dimensional
- MD:
-
Molecular dynamics
References
Dennis EA, Rhee SG, Billah MM, Hannun YA, (1991) Faseb J 5(7):2068–2077
Bennion C, Connolly S, Gensmantel NP, Hallam C, Jackson CG, Primrose WU, Roberts GC, Robinson DH, Slaich PK (1992) J Med Chem 35(16):2939–2951
Jackson RC (1995) Curr Opin Biotechnol 6(6):646–651
Ortiz AR, Pisabarro MT, Gago F, Wade RC (1995) J Med Chem 38(14):2681–2691
Lindahl M, Tagesson C (1997) Inflammation 21(3):347–356
Chemical Computing Group I (2006) Montreal, Quebec, Canada H3A 2R7
Allen FH (2002) Acta Crystallogr B 58(Pt 3 Pt 1):380–388
Koopmans T (1933) Physica 1:104–113
Stewart JJP (1990) J Comp Aid Mol Des 1(4):1–45
Gester S, Metz P, Zierau O, Vollmer G (2001) Tetrahedron 57(6):1015–1018
Tischer S, Metz P (2007) Adv Synth Catal 349(1–2):147–151
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) Nucleic Acids Res 28(1):235–242
Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL (2000) Mol Cell 6(4):909–919
Steiner RA, Kalk KH, Dijkstra BW (2002) Proc Natl Acad Sci USA 99(26):16625–16630
Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP (1999) Nat Struct Biol 6(8):775–784
Jez JM, Bowman ME, Dixon RA, Noel JP (2000) Nat Struct Biol 7(9):786–791
Welford RW, Clifton IJ, Turnbull JJ, Wilson SC, Schofield CJ (2005) Org Biomol Chem 3(17):3117–3126
Hansford KA, Reid RC, Clark CI, Tyndall JD, Whitehouse MW, Guthrie T, McGeary RP, Schafer K, Martin JL, Fairlie DP (2003) Chembiochem 4(2–3):181–185
Pan YH, Yu BZ, Berg OG, Jain MK, Bahnson BJ (2002) Biochemistry 41(50):14790–14800
BioSolveIT G (2007) An der Ziegelei 75:53757 St. Augustin, Germany
Schevitz RW, Bach NJ, Carlson DG, Chirgadze NY, Clawson DK, Dillard RD, Draheim SE, Hartley LW, Jones ND, Mihelich ED et al (1995) Nat Struct Biol 2(6):458–465
Case CA, Darden TA, Cheatham ITE, Simmerling CL, Wang J, Duke RE, Luo R, Merz KM, Wang B, Pearlman DA, Crowley M, Brozell S, Tsui V, Gohlke H, Mongan J, Hornak H, Cui G, Beroza P, Schafmeister C, Caldwell JW, Ross WS, Kollman PA (2004) Amber8 users manual, University of California, San Francisco
Humphrey W, Dalke A, Schulten K (1996) J Mol Graph 14:33–38
Bohl M, Ketscher L, Tietbohl C, Gutzeit HO (2006) Anal Biochem 359(2):280–282
Leslie CC, Gelb MH (2004) Methods Mol Biol 284:229–242
Menschikowski M, Hagelgans A, Heyne B, Hempel U, Neumeister V, Goez P, Jaross W, Siegert G (2005) Biochim Biophys Acta 1733(2–3):157–171
Pisabarro MT, Ortiz AR, Palomer A, Cabre F, Garcia L, Wade RC, Gago F, Mauleon D, Carganico G (1994) J Med Chem 37(3):337–341
Guerrero JA, Lozano ML, Castillo J, Benavente-Garcia O, Vicente V, Rivera JJ (2005) Thromb Haemost 3(2):369–376
van Zanden JJ, Geraets L, Wortelboer HM, van Bladeren PJ, Rietjens IM, Cnubben NH (2004) Biochem Pharmacol 67(8):1607–1617
Prout CK, Wallwork SC (1966) Acta Crystallogr 21:449–450
Gil B, Sanz MJ, Terencio MC, Ferrandiz ML, Bustos G, Paya M, Gunasegaran R, Alcaraz MJ (1994) Life Sci 54(20):PL333–PL338
Chang HW, Baek SH, Chung KW, Son KH, Kim HP, Kang SS (1994) Biochem Biophys Res Commun 205(1):843–849
Acknowledgments
This work has been funded by European Structural Funds (EFRE). MTP group is funded by the Klaus Tschira Stiftung GmbH. We are grateful to Mrs. Mandy Erlitz for her invaluable administrative support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lättig, J., Böhl, M., Fischer, P. et al. Mechanism of inhibition of human secretory phospholipase A2 by flavonoids: rationale for lead design. J Comput Aided Mol Des 21, 473–483 (2007). https://doi.org/10.1007/s10822-007-9129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-007-9129-8