Abstract
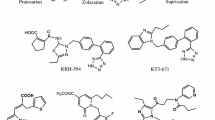
A new 1,5 disubstituted imidazole AT1 Angiotensin II (AII) receptor antagonist related to losartan with reversion of butyl and hydroxymethyl groups at the 2-, 5-positions of the imidazole ring was synthesized and evaluated for its antagonist activity (V8). In vitro results indicated that the reorientation of butyl and hydroxymethyl groups on the imidazole template of losartan retained high binding affinity to the AT1 receptor concluding that the spacing of the substituents at the 2,5- positions is of primary importance. The docking studies are confirmed by binding assay results which clearly show a comparable binding score of the designed compound V8 with that of the prototype losartan. An efficient, regioselective and cost effective synthesis renders the new compound as an attractive candidate for advanced toxicological evaluation and a drug against hypertension.






Similar content being viewed by others
References
Ferrario CM (1990) J Cardiovasc Pharmacol 15:51–55
Corvol P (1989) Clin Exp Hypertens 2:463–470
Berecek KH, King SJ, Wu JN (1993) CRC Press: Boca Raton, 183–220
Waeber B, Nussberger J, Brunner HR (eds.) (1990) Raven Press: New York, 2209–2232
Lindgren BR, Andersson RG (1989) Med Toxicol Adverse Drug Exp 4:369–380
Wexler RR, Greenlee WJ, Irvin JD, Goldberg MR, Prendergast K, Smith RD, Timmermans PBMWM (1996) J Med Chem 39:625–655
De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T (2000) Pharmacol Rev 52:415–472
Moore G, Smith J, Baylis B, Matsoukas J (1995) Adv Pharmacol 6:91–141
Giannis A, Bubsam F (1997) Adv Drug Res 29:1–78
Duncia JV, Carini DJ, Chiu AT, Pierce ME, Price WA, Smith RD, Wells GJ, Wong PC, Wexler RR, Johnson AL, Timmermans PBMWM (1992) Drugs Future 17:326–331
Ashton WT (1994) Exp Opin Invest Drugs 3:1105–1142
Buhlmayer P (1992) Curr Opin Ther Pat 2:1693–1718
Bradbury RH, Allot CP, Dennis M, Fisher E, Major JS, Masek BB, Oldham AA, Russell ST (1992) J Med Chem 35:4027–4038
Masek BB, Merchant A, Matthew JB (1993) J Med Chem 36:1230–1238
Easthope SE, Jarvis B (2002) Drugs 62:1253–1287
Rabbat CG (2002) ACP J Club 136:82–84
Cheng-Lai A (2002) Heart Dis 4:54–59
Maillard MP, Rossat J, Brunner HR, Burnier MJ (2000) Pharmacol Exp Ther 295:649–654
Brunner HR, Gavras H (2002) Lancet 359:990–992
Ismail MAH, Barker S, Abou el-Ella DA, Abouzid KAM, Toubar RA, Todd MH (2006) J Med Chem 49:1526–1535
Cappelli A, Nannicini C, Gallelli A, Giuliani G, Valenti S, Mohr GP, Anzini M, Mennuni L, Ferrari F, Caselli G, Girdani A, Peris W, Makovec F, Giorgi G, Vomero S (2008) J Med Chem 51:2137–2146
Theodoropoulou E, Mavromoustakos T, Panagiotopoulos D, Matsoukas JM, Smith J (1996) Lett Pept Sci 3:209–215
Polevaya L, Mavromoustakos T, Zoumpoulakis P, Crdadolnik S, Roumelioti P, Giatas N, Mutule I, Vlahakos D, Iliodromitis E, Kremastinos D, Matsoukas J (2001) Bioorg Med Chem 9:1639–1647
Moore GJ, Ganter RC, Matsoukas JM, Hondrelis J, Agelis G, Barlos K, Wilkinson S, Sandall J, Fowler P (1994) J Mol Rec 7:251–256
Turner RJ, Matsoukas JM, Moore GJ (1991) Biochim Biophys Acta 1065:21–28
Matsoukas JM, Agelis G, Hondrelis J, Yamdagni R, Wu Q, Ganter R, Smith J, Moore D, Moore GJ (1993) J Med Chem 36:904–911
Matsoukas JM, Yamdagni R, Moore GJ (1990) Peptides 11:367–374
Mavromoustakos T, Kolocouris A, Zervou M, Roumelioti P, Matsoukas J, Weisemann R (1999) J Med Chem 42:1714–1722
Zoumpoulakis P, Politi A, Grdadolnik SG, Matsoukas J, Mavromoustakos T (2006) J Pharm Biomed Anal 40:1097–1104
Wahhab A, Smith JR, Ganter RC, Moore DM, Hondrelis J, Matsoukas J, Moore GJ (1993) Arzn.-Forsch./Drug Res 43:1157–1168
Tuccinardi T, Calderone V, Rapposelli S, Martinelli A (2006) J Med Chem 49:4305–4316
Okada T, Sugihara M, Bondar AN, Elstner M, Entel P, Buss V (2004) J Mol Biol 342:571–583
Jones G, Willett P, Glen RC, Leach AR, Taylor R (1997) J Mol Biol 267:727–748
Friesner RA, Murphy RB, Repasky MP, Frye LL, Greenwood JR, Halgren TA, Sanschagrin PC, Mainz DT (2006) J Med Chem 49:6177–6196
Sherman W, Day T, Jacobson MP, Friesner RA, Farid R (2006) J Med Chem 49:534–553
Schrodinger, LLC: Portland, OR, 2007, Web address: www.schrodinger.com
Jones G, Willett P, Glen RC (1995) J Mol Biol 245:43–53
Judson S, Jaeger EP, Treasurywala AM (1994) J Mol Struct 308:191–206
Oshiro CM, Kuntz ID, Dixon JS (1995) J Comput -Aided Mol Des 9:113–130
GOLD user manual, web address: www.ccdc.cam.ac.uk
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) J Comput Chem 30:2785–2791
Meyers AI, Mihelich ED (1975) J Am Chem Soc 97:7383–7385
Dordor IM, Mellor JM (1983) Tetrahedron Lett 24:1437–1440
Duncia JV, Pierce ME, Santella JB (1991) J Org Chem 56:2395
Noskov SY (2008) Proteins 73(4):851–863
Barlos K, Papaioannou D, Theodoropoulos D (1982) J Org Chem 47:1324–1326
Athanasopoulos C, Balayiannis G, Karigiannis G, Papaioannou DA (1999) IOS Press 22:137–151
Athanassopoulos P, Barlos K, Gatos D, Hatzi O, Tzavara C (1995) Tetrahedron Lett 36:5645–5648
Barlos K, Gatos D (1999) Biopolymers 51:266–278
Krambovitis E, Hatzidakis G, Barlos K (1998) J Biol Chem 273:10874–10879
Lauth-de Viguerie N, Sergueeva N, Damiot M, Mawlawi H, Riviere M, Lattes A (1994) Heterocycles 37:1561–1578
Matsoukas JM, Hondrelis J, Keramida M, Mavromoustakos T, Makriyannis A, Yamdagni R, Wu Q, Moore J (1994) J Biol Chem 269:5303–5312
Matsoukas JM, Agelis G, Wahhab A, Hondrelis J, Panagiotopoulos D, Yamdagni R, Wu Q, Mavromoustakos T, Maia HLS, Ganter R, Moore GJ (1995) J Med Chem 38:4660–4669
Matsoukas JM, Cordopatis P, Belte U, Goghari MH, Ganter RC, Franklin KJ, Moore GJ (1998) J Med Chem 31:1418–1421
Matsoukas JM, Goghari MA, Scanlon MN, Franklin KJ, Moore GJ (1985) J Med Chem 28:780–783
Matsoukas J, Hondrelis J, Agelis G, Barlos K, Gatos D, Ganter R, Moore D, Moore G (1994) J Med Chem 37:2958–2969
Polevaya L, Mavromoustakos T, Zoumboulakis P, Grdadolnik SG, Roumelioti P, Giatas N, Mutule I, Keivish T, Vlahakos DV, Iliodromitis EK, Kremastinos DT, Matsoukas J (2001) Bioorg Med Chem 6:1639–1647
Vlahakos DV, Matsoukas JM, Ancans J, Moore GJ, Iliodromitis EK, Marathias KP, Kremastinos D (1996) Lett Pept Sci 3:191–194
Matsoukas J, Ancas J, Mavromoustakos T, Kolocouris A, Roumelioti P, Vlahakos D, Yamdagni R, Wu Q, Moore G (2000) Bioorg Med Chem 8:1–10
Roumelioti P, Polevaya L, Mavromoustakos T, Zoumboulakis P, Giatas N, Mutule I, Keivish T, Zoga A, Vlahakos DV, Iliodromitis EK, Kremastinos DT, Matsoukas J (2002) Bioorg Med Chem 12:2627–2633
Potamitis C, Zervou M, Katsiaras V, Zoumpoulakis P, Durdagi S, Papadopoulos M, Hayes J, Grdadolnik S, Kyrikou I, Argyropoulos D, Vatougia G, Mavromoustakos T (2009) J Chem Inf Mod 49:726–729
Acknowledgments
This work was supported by grants from the Ministry of Development of Greece, General Secretariat of Research and Technology, EPET II, 115/PENED, 1999/EPAN, 114 from the Ministry of Education (postgraduate program “Medicinal Chemistry” EPEAEK). We also acknowledge NATO (Linkage Grant 974548). We also acknowledge Prof. J. Findlay and Dr. Alan Cox from School of Biochemistry and Microbiology, University of Leeds, Leeds, LS2 9JT, UK who kindly provided the laboratory facilities to Prof. T. Mavromoustakos a recipient of Royal Society scholarship. We also acknowledge A. Suarez for her linguistic amendment of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Docking Studies: Serdar Durdagi, Thomas Mavromoustakos
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Agelis, G., Roumelioti, P., Resvani, A. et al. An efficient synthesis of a rationally designed 1,5 disubstituted imidazole AT1 Angiotensin II receptor antagonist: reorientation of imidazole pharmacophore groups in losartan reserves high receptor affinity and confirms docking studies. J Comput Aided Mol Des 24, 749–758 (2010). https://doi.org/10.1007/s10822-010-9371-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-010-9371-3