Abstract
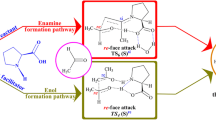
In this study the formation of the lactyl–thiamin diphosphate intermediate (L–ThDP) is addressed using density functional theory calculations at X3LYP/6-31++G(d,p) level of theory. The study includes potential energy surface scans, transition state search, and intrinsic reaction coordinate calculations. Reactivity is analyzed in terms of Fukui functions. The results allow to conclude that the reaction leading to the formation of L–ThDP occurs via a concerted mechanism, and during the nucleophilic attack on the pyruvate molecule, the ylide is in its AP form. The calculated activation barrier for the reaction is 19.2 kcal/mol, in agreement with the experimental reported value.












Similar content being viewed by others
References
McCourt JA, Duggleby RG (2006) Acetohydroxyacid synthase and its role in the biosynthetic pathway for branched-chain amino acids. Amino Acids 31:173–210
Paramasivam S, Balakrishnan A, Dmitrenko O, Godert A, Begley TP, Jordan F, Polenova T (2011) Solid-state NMR and density functional theory studies of ionization sates of thiamin. J Phys Chem B 115:730–736
Kern D, Kern G, Neef H, Tittmann K, Killenberg-Jabs M, Wikner C, Schneider G, Hübner G (1997) How thiamin diphosphate is activated in enzymes? Science 275:67–70
Tittmann K, Golbik R, Uhlemann K, Khailova L, Schneider G, Patel M, Jordan F, Chipman DM, Dugglebly RG, Hübner G (2003) NMR analysis of covalent intermediates in thiamin diphosphate enzymes. Biochemistry 42:7885–7891
Friedemann R, Tittmann K, Golbik R, Hübner G (2009) DFT and MP2 studies on the C2–C2α bond cleavage in thiamin catalysis. J Mol Catal B Enzym 61:36–38
Chipman DM, Duggleby RG, Tittmann K (2005) Mechanisms of acetohydroxyacid synthases. Curr Opin Chem Biol 9:475–481
Xiong Y, Liu J, Yang GF, Zhan CG (2010) Computational determination of fundamental pathways and activation barriers for acetohydroxyacid synthase-catalyzed condensation reactions of α-keto acids. J Comput Chem 31:1592–1602
Xu X, Goddard WA III (2004) The X3LYP extended density functional for accurate descriptions of nonbond interactions, spin states, and thermochemical properties. Proc Natl Acad Sci USA 101:2673–2677
Delgado EJ, Alderete JB, Jaña GA (2011) Density-functional study on the equilibria in the ThDP activation. J Mol Model 17:2735–2739
Schrodinger (2010) Jaguar, version 7.7. Schrodinger, LLC, New York
Jordan F, Nemeria NS (2005) Experimental observation of thiamin diphosphate-bound intermediates on enzymes and mechanistic information derived from these observations. Bioorg Chem 33:190–215
Nemeria NS, Chakraborty S, Balakrishnan A, Jordan F (2009) Reaction mechanisms of thiamin diphosphate enzymes: defining states of ionization and tautomerization of the cofactor at individual steps. FEBS J 276:2432–2446
Nemeria N, Chakraborty S, Baykal A, Korotchkina LG, Patel MS, Jordan F (2007) The 1′,4′-iminopyrimidine tautomer of thiamin diphosphate is poised for catalysis in asymmetric active centers on enzymes. Proc Natl Acad Sci USA 104:78–82
Acknowledgments
The authors gratefully acknowledge financial support from Fondecyt, Grant No. 1100064.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Alvarado, O., Jaña, G. & Delgado, E.J. Computer-assisted study on the reaction between pyruvate and ylide in the pathway leading to lactyl–ThDP. J Comput Aided Mol Des 26, 977–982 (2012). https://doi.org/10.1007/s10822-012-9589-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-012-9589-3