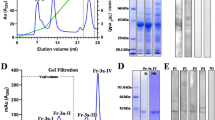
Abstract
Isoflavone reductase-like proteins (IRLs) are enzymes with key roles in the metabolism of diverse flavonoids. Last identified olive pollen allergen (Ole e 12) is an IRL relevant for allergy amelioration, since it exhibits high prevalence among atopic patients. The goals of this study are the characterization of (A) the structural-functionality of Ole e 12 with a focus in its catalytic mechanism, and (B) its molecular allergenicity by extensive analysis using different molecular computer-aided approaches covering (1) physicochemical properties and functional-regulatory motifs, (2) sequence analysis, 2-D and 3D structural homology modeling comparative study and molecular docking, (3) conservational and evolutionary analysis, (4) catalytic mechanism modeling, and (5) sequence, structure-docking based B-cell epitopes prediction, while T-cell epitopes were predicted by inhibitory concentration and binding score methods. Structural-based detailed features, phylogenetic and sequences analysis have identified Ole e 12 as phenylcoumaran benzylic ether reductase. A catalytic mechanism has been proposed for Ole e 12 which display Lys133 as one of the conserved residues of the IRLs catalytic tetrad (Asn-Ser-Tyr-Lys). Structure characterization revealed a conserved protein folding among plants IRLs. However, sequence polymorphism significantly affected residues involved in the catalytic pocket structure and environment (cofactor and substrate interaction-recognition). It might also be responsible for IRLs isoforms functionality and regulation, since micro-heterogeneities affected physicochemical and posttranslational motifs. This polymorphism might have large implications for molecular differences in B- and T-cells epitopes of Ole e 12, and its identification may help designing strategies to improve the component-resolving diagnosis and immunotherapy of pollen and food allergy through development of molecular tools.







Similar content being viewed by others
References
Moummou H, Kallberg Y, Tonfack LB, Persson B, van der Rest B (2012) BMC Plant Biol 12:219
Banerjee S, Li Y, Wang Z, Sarkar FH (2008) Cancer Lett 269:226–242
Lewis NG, Davin LB (1999) In: Barton DHR, Nakanishi K, Meth-Cohn O (eds) Comprehensive natural products chemistry. Elsevier, London
Dixon RA (1999) In: Sankawa U (ed) Comprehensive natural products chemistry. Elsevier, Oxford
Taylor LP, Grotewold E (2005) Curr Opin Plant Biol 8:317–323
Buer CS, Imin N, Djordjevic MA (2010) J Integr Plant Biol 52:98–111
van Eldik GJ, Ruiter RK, Colla PH, van Herpen MM, Schrauwen JA, Wullems GJ (1997) Plant Mol Biol 33:923–929
Vieths S, Frank E, Scheurer S, Meyer HE, Hrazdina G, Haustein D (1998) Scand J Immunol 47:263–267
Lers A, Burd S, Lomaniec E, Droby S, Chalutz E (1998) Plant Mol Biol 36:847–856
Gang DR, Kasahara H, Xia ZQ, Mijnsbrugge KV, Bauw G, Boerjan W, Van Montagu M, Davin LB, Lewis NG (1999) J Biol Chem 274:7516–7527
Karamloo F, Schmitz N, Scheurer S, Foetisch K, Hoffmann A, Haustein D, Vieths S (1999) J Allergy Clin Immunol 104(5):991–999
Suzuki S, Umezawa T (2007) J Wood Sci 53:273–284
Dinkova-Kostova AT, Gang DR, Davin LB, Bedger DL, Chu A, Lewis NG (1996) J Biol Chem 271:29473–29482
Fujita M, Gang DR, Davin LB, Lewis NG (1999) J Biol Chem 274:618–627
Paiva NL, Edwards R, Sun Y, Hrazdina G, Dixon RA (1991) Plant Mol Biol 17:653–667
Tiemann K, Inzé D, Van Montagu M, Barz W (1991) Eur J Biochem 200:751–757
Paiva NL, Sun Y, Dixon RA, Van Etten HD, Hrazdina G (1994) Arch Biochem Biophys 312:501–510
Wang X, He X, Lin J, Shao H, Chang Z, Dixon RA (2006) J Mol Biol 358:1341–1352
Min T, Kasahara H, Bedgar DL, Youn B, Lawrence PK, Gang DR, Halls SC, Park H, Hilsenbeck JL, Davin LB, Lewis NG, Kang C (2003) J Biol Chem 278:50714–50723
Will-Karp M, Santeliz J, Karp CL (2001) Nat Rev Immunol 1:69–75
Blanca M, Boulton P, Brostoff J, González-Reguera I (1983) Clin Allergy 13:473–478
Hamman-Khalifa A, Castro AJ, Jimenez-Lopez JC, Rodríguez-García MI, Alché JD (2008) BMC Plant Biol 8:10
Jimenez-Lopez JC, Morales S, Castro AJ, Volkmann D, Rodríguez-García MI, Alché JD (2012) PLoS ONE 7(2):e30878
Jimenez-Lopez JC, Rodríguez-García MI, Alché JD (2013) PLoS ONE 8(10):e76066. doi:10.1371/journal.pone.0076066
Salamanca G, Rodriguez R, Quiralte J, Moreno C, Pascual CY, Barber D, Villalba M (2010) FEBS J 277:2729–2739
Jimenez-Lopez JC, Kotchoni SO, Rodríguez-García MI, Alché JD (2012) J Mol Model 18:4965–4984
Wu S, Zhang Y (2010) Structure 18:858–867
Kozakov D, Hall DR, Beglov D, Brenke R, Comeau SR, Shen Y, Li K, Zheng J, Vakili P, Paschalidis IC, Vajda S (2010) Proteins 78:3124–3130
Kyte J, Doolittle RF (1982) J Mol Biol 157:105–132
Welling GW, Weijer WJ, van der Zee R, Welling-Wester S (1985) FEBS Lett 188:215–218
Parker JMR, Guo D, Hodges RS (1986) Biochemistry 25:5425–5431
Hopp TP, Woods KR (1981) Proc Natl Acad Sci USA 78:3824–3828
Esteve C, Montealegre C, Marina ML, Garcia MC (2012) Talanta 92:1–14
Louie GV, Baiga TJ, Bowman ME, Koeduka T, Taylor JH et al (2007) PLoS ONE 2:e993
Levin I, Schwarzenbacher R, McMullan D, Abdubek P, Ambing E et al (2004) Proteins 56:629–633
Arcangeli C, Cantale C, Galeffi P, Rosato V (2008) J Struct Biol 164:119–133
Bovy A, de Vos R, Kemper M, Schijlen E, Almenar M, Muir S, Collins G, Robinson S, Verhoeyen M, Hughes S, Santos C, van Tunen A (2002) Plant Cell 14:2509–2526
Kim SS, Grienenberger E, Lallemand B, Colpitts CC, Kim SY, Souza A, Geoffroy P, Heintz D, Krahn D, Kaiser M, Kombrink E, Heitz T, Suh DY, Legrand M, Douglas CJ (2010) Plant Cell 22:4045–4066
Tanaka N, Nonaka T, Nakanishi M, Deyashiki Y, Hara A, Mitsui Y (1996) Structure 4:33–45
Shao H, Dixon RA, Wang X (2007) J Mol Biol 369:265–276
Shimada N, Sato S, Akashi T, Nakamura Y, Tabata S, Ayabe S-I, Aoki T (2007) DNA Res 14:25–36
Gutierrez-Gonzalez JJ, Wu X, Gillman JD, Lee JD, Zhong R, Yu O, Shannon G, Ellersieck M, Nguyen HT, Sleper DA (2010) BMC Plant Biol 10:105
Jornvall H, Persson B, Krook M, Atrian S, Gonzalez-Duarte R, Jeffery J, Ghosh D (1995) Biochem 34:6003–6013
Oppermann U, Filling C, Hult M, Shafqat N, Wu X, Lindh M, Shafqat J, Nordling E, Kallberg Y, Persson B, Jornvall H (2003) Chem Biol Interact 143–144:247–253
Lesk AM (1995) Curr Opin Struct Biol 5:775–783
Grimm C, Maser E, Mobus E, Klebe G, Reuter K, Ficner R (2000) J Biol Chem 275:41333–41339
Ghosh D, Sawicki M, Pletnev V, Erman M, Ohno S, Nakajin S, Duax WL (2001) J Biol Chem 276:18457–18463
Zubieta C, Ross JR, Koscheski P, Yang Y, Pichersky E, Noel JP (2003) Plant Cell 15:1704–1716
Sakuraba H, Kawai T, Yoneda K, Ohshima T (2011) Arch Biochem Biophys 512(2):126–134
Kavanagh KL, Jörnvall H, Persson B, Oppermann U (2008) Cell Mol Life Sci 65(24):3895–3906
Karamloo F, Wangorsch A, Kasahara H, Davin LB, Haustein D, Lewis NG, Vieths S (2001) Eur J Biochem 268:5310–5320
Wang P, Sidney J, Dow C, Mothe B, Sette A, Peters B (2008) PLoS Comput Biol 4:e1000048
Dall’Antonia F, Gieras A, Devanaboyina SC, Valenta R, Keller W (2011) J Allergy Clin Immunol 128(4):872–879
García-Casado G, Pacios LF, Díaz-Perales A, Sánchez-Monge R, Lombardero M, García-Selles FJ, Polo F, Barber D, Salcedo G (2003) J Allergy Clin Immunol 112(3):599–605
Ball T, Fuchs T, Sperr WR, Valent P, Vangelista L, Kraft D, Valenta R (1999) FASEB J 13:1277–1290
Midoro-Horiuti T, Schein CH, Mathura V, Braun W, Czerwinski EW, Togawa A, Kondo Y, Oka T, Watanabe M, Goldbluma RM (2006) Mol Immunol 43(6):509–518
Jimenez-Lopez JC, Gachomo WE, Ariyo O, Baba-Moussa L, Kotchoni SO (2012) Mol Biol Rep 39(1):123–130
Radauer C, Willerroiderw M, Fuchs H, Hoffmann K, Thalhamerw J, Ferreira F, Scheiner O, Breiteneder H (2006) Clin Exp Allergy 36:920–929
Jahn-Schmid B, Kelemen P, Himly M, Bohle B, Fischer G, Ferreira F, Ebner C (2002) J Immunol 169:6005–6011
Oezguen N, Zhou B, Negi SS, Ivanciuc O, Schein CH, Labesse G, Braun W (2008) Mol Immunol 45:3740–3747
Wolff N, Yannai S, Karin N, Levy Y, Reifen R, Dalal I, Cogan U (2004) J Allergy Clin Immunol 114:1151–1158
Brinda KV, Vishveshwara S (2005) BMC Bioinformatics 6:296
Sinha N, Mohan S, Lipschultz CA, Smith-Gill SJ (2002) Biophys J 83:2946–2968
Karamloo F, Schmitz N, Scheurer S, Foetisch K, Hoffmann A, Haustein D, Vieths S (1999) J Allergy Clin Immunol 104:991–999
Custovic A, Nicolaou N (2011) J Allergy Clin Immunol 127:631–632
Bock SA, Munoz-Furlong A, Sampson HA (2007) J Allergy Clin Immunol 119:1016–1018
Mittag D, Akkerdaas J, Ballmer-Weber BK, Vogel L, Wensing M, Becker WM, Koppelman SJ, Knulst AC, Helbling A, Hefle SL, Van Ree R, Vieths S (2004) J Allergy Clin Immunol 114:1410–1417
Nicolaou N, Custovic A (2011) Curr Opin Allergy Clin Immunol 11:222–228
Asarnoj A, Nilsson C, Lidholm J, Glaumann S, Ostblom E, Hedlin G, van Hage M, Lilja G, Wickman M (2012) J Allergy Clin Immunol 130(2):468–472
Levy DA, Charpin D, Pecquet C, Leynadier F, Vervtoet D (1992) Allergy 47:579–587
Düring K (1993) Plant Mol Biol 23:209–214
Chen Z, Cremer R, Posch A, Raulf-Heimsoth M, Rihs HP, Baur X (1997) J Allergy Clin Immunol 100:684–693
Chen Z, van Kampen V, Raulf-Heimsoth M, Baur X (1996) Clin Exp Allergy 26:406–415
Schuler S, Ferrari G, Schmid-Grendelmeier P, Harr T (2013) Clin Transl Allergy 3(1):11
Wagner S, Breiteneder H (2002) Biochem Soc Trans 30(6):935–940
Wopfner N, Gadermaier G, Egger M, Asero R, Ebner C, Jahn-Schmid B, Ferreira F (2005) Int Arch Allergy Immunol 138(4):337–346
Jahn-Schmid B, Hauser M, Wopfner N, Briza P, Berger UE, Asero R, Ebner C, Ferreira F, Bohle B (2012) J Immunol 188(3):1559–1567
Jornvall H, Persson M, Jeffery J (1981) Proc Natl Acad SciUSA 78:4226–4230
Hoffmann F, Maser E (2007) Drug Metab Rev 39:87–144
Kallberg Y, Oppermann U, Jornvall H, Persson B (2002) Protein Sci 11:636–641
Mauge C, Granier T, Langlois d’Estaintot B, Gargouri B, Manigand C, Schmitter JM, Chaudière J, Gallois B (2010) J Mol Biol 397:1079–1091
Filling C, Berndt KD, Benach J, Knapp S, Prozorovski T, Nordling E, Ladenstein R, Jornvall H, Oppermann U (2002) J Biol Chem 277(28):25677–25684
Swanson BA, Frey PA (1993) Biochem 32:13231–13236
Tohge T, Watanabe M, Hoefgen R, Fernie AR (2013) Crit Rev Biochem Mol Biol 48(2):123–152
Limem I, Guedon E, Hehn A, Bourgaud F, Chekir Ghedira L, Engasser JM, Ghoul M (2008) Process Biochem 43:463–479
Liu CJ, Blount JW, Steele CL, Dixon RA (2002) Proc Natl Acad Sci USA 99:14578–14583
Julien Racle JO, Hatzimanikatis V (2012) Biotechnol Bioeng 109:2127–2133
Brunk E, Neri M, Tavernelli I, Hatzimanikatis V, Rothlisberger U (2012) Biotechnol Bioeng 109:572–582
Soh KC, Hatzimanikatis V (2010) Trends Biotechnol 28:501–508
Miskovic L, Hatzimanikatis V (2010) Trends Biotechnol 28:391–397
Xu P, Bhan N, Koffas MAG (2013) Curr Opin Biotechnol 24(2):291–299
Kim MI, Kwon SJ, Dordick JS (2009) Org Lett 11:3806–3809
Kwon SJ, Lee M-Y, Ku B, Sherman DH, Dordick JS (2007) ACS Chem Biol 2:419–425
Bhan N, Xu P, Koffas MAG (2013) Curr Opin Biotechnol. doi:10.1016/j.copbio.2013.02.019
Acknowledgments
This study was supported by the following European Regional Development Fund co-financed Grants: MCINN BFU 2004-00601/BFI, BFU 2008-00629, BFU2011-22779, CICE (Junta de Andalucía) P2010-CVI15767, P2010-AGR6274 and P2011-CVI-7487, and by the coordinated project Spain/Germany MEC HA2004-0094. JCJ-L thanks Spanish CSIC and the European Marie Curie research program (FP7-PEOPLE-2011-IOF) for his I3P-BPD-CSIC and PIOF-GA-2011-301550 Grants, respectively.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10822_2013_9686_MOESM1_ESM.pdf
Figure S1. Ole e 12 modeling assessment. (a) Ramachandran plot of the modeled Ole e 12 protein. (b) ‘Z-plot’ and (c) ‘e-plot’ of Ole e 12 protein model. (d) Energy level distribution representation in the Ole e 12 protein model. The amino acid colors are clamped at red (highest energy) or blue (lowest energy). Supplementary material 1 (PDF 117 kb)
10822_2013_9686_MOESM2_ESM.pdf
Figure S2. Chemical structures of flavonoids. a) Diagram of the natural polyphenol classification. b) Examples of pollen polyphenols (flavonoids) with significant antioxidant activity by substitution of radicals R1, R2, R3, R4 and R5. c) Basic structures of flavonoid subclasses. Supplementary material 2 (PDF 73 kb)
10822_2013_9686_MOESM3_ESM.pdf
Figure S3. Diagram representation of highly antigenicity regions in olive Ole e 12 protein. Eight areas of high antigenicity are highlighted with gray color shadows, as a result of the combination of paramenters such as hydrophobicity (or hydrophilicity), Kyte-Doolitte scale and Hopp-Woods scale; antigenicity, Welling method and Parker method. Surface accessibility of amino acids, 30% and 50% (discontinue blue and red line, respectively). Supplementary material 3 (PDF 156 kb)
Glossary
- Å
-
Armstrong
- ANOLEA
-
Atomic non-local environment assessment
- ASA
-
Absolute surface area
- Cα
-
Carbon alpha
- CATH
-
Protein structure classification
- CDART
-
Conserved domain architecture retrieval tool
- CDD
-
Conserved domain database
- ExPASy
-
Expert protein analysis system
- FASTA
-
Fast alignment
- FFT
-
Fast fourier transform
- G-factor
-
Goodness factor
- GETAREA
-
Solvent accessible surface area or solvation energy
- GO terms
-
The gene ontology project
- GRAVY
-
Grand average of hydropathicity
- GROMOS96
-
Force field for molecular dynamics simulation
- HLA
-
Human leukocyte antigen
- IC50
-
Inhibitory concentration
- IFR
-
Isoflavone reductase
- IRLs
-
Isoflavone reductase-like proteins
- Jscore
-
Jury score
- MHC
-
Major histocompatibility complex
- NCBI
-
National center for biotechnology information
- NJ
-
Neighbor-joining
- NMR
-
Nuclear magnetic resonance
- PB
-
Electrostatic Poisson-Boltzmann
- PCBER
-
Phenylcoumaran benzylic ether reductase
- PDB
-
Protein data bank
- PIP-Family
-
PCBER, IFR and PRL family
- PIRSF
-
Family classification system at the protein information resource
- PLR
-
Pinoresinol-lariciresinol reductase
- ProSa
-
Protein structure analysis
- PROSITE
-
Database of protein domains, families and functional sites
- PROCHECK
-
Protein structure validation server
- QMEAN
-
Protein model quality estimation server
- SASA
-
Solvent accessible surface areas
- SDR
-
Short-chain dehydrogenase/reductase
- SDAP
-
Structural database of allergenic proteins
- SMART
-
Simple modular architecture research tool
- RMSD
-
Root mean square deviation
Rights and permissions
About this article
Cite this article
Jimenez-Lopez, J.C., Kotchoni, S.O., Hernandez-Soriano, M.C. et al. Structural functionality, catalytic mechanism modeling and molecular allergenicity of phenylcoumaran benzylic ether reductase, an olive pollen (Ole e 12) allergen. J Comput Aided Mol Des 27, 873–895 (2013). https://doi.org/10.1007/s10822-013-9686-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-013-9686-y