Abstract
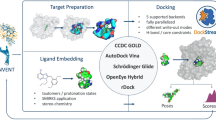
Structure-based drug design (SBDD) has matured within the last two decades as a valuable tool for the optimization of low molecular weight lead compounds to highly potent drugs. The key step in SBDD requires knowledge of the three-dimensional structure of the target-ligand complex, which is usually determined by X-ray crystallography. In the absence of structural information for the complex, SBDD relies on the generation of plausible molecular docking models. However, molecular docking protocols suffer from inaccuracies in the description of the interaction energies between the ligand and the target molecule, and often fail in the prediction of the correct binding mode. In this context, the appropriate selection of the most accurate docking protocol is absolutely relevant for the final molecular docking result, even if addressing this point is absolutely not a trivial task. D3R Grand Challenge 2015 has represented a precious opportunity to test the performance of DockBench, an integrate informatics platform to automatically compare RMDS-based molecular docking performances of different docking/scoring methods. The overall performance resulted in the blind prediction are encouraging in particular for the pose prediction task, in which several complex were predicted with a sufficient accuracy for medicinal chemistry purposes.







Similar content being viewed by others
References
Directory of in silico Drug Design tools—Docking. http://www.click2drug.org/directory_Docking.html. Accessed 25 May 2016
Docking (molecular)—Wikipedia. https://en.wikipedia.org/wiki/Docking_(molecular). Accessed 25 May 2016
Cuzzolin A, Sturlese M, Malvacio I, Ciancetta A, Moro S (2015) DockBench: an integrated informatic platform bridging the gap between the robust validation of docking protocols and virtual screening simulations. Molecules 20:9977–9993. doi:10.3390/molecules20069977
Solit DB, Rosen N (2006) Hsp90: a novel target for cancer therapy. Curr Top Med Chem 6:1205–1214. doi:10.2174/156802606777812068
Virbasius JV, Czech MP (2016) Map4k4 signaling nodes in metabolic and cardiovascular diseases. Trends Endocrinol Metab. doi:10.1016/j.tem.2016.04.006
Harvey MJ, Giupponi G, Fabritiis GD (2009) ACEMD: accelerating biomolecular dynamics in the microsecond time scale. J Chem Theory Comput 5:1632–1639. doi:10.1021/ct9000685
Masciocchi J, Frau G, Fanton M, Sturlese M, Floris M, Pireddu L, Palla P, Cedrati F, Rodriguez-Tomé P, Moro S (2009) MMsINC: a large-scale chemoinformatics database. Nucleic Acids Res 37:D284–D290. doi:10.1093/nar/gkn727
Sadowski J, Gasteiger J, Klebe G (1994) Comparison of automatic three-dimensional model builders using 639 X-ray structures. J Chem Inf Comput Sci 34:1000–1008
Labute P (2009) Protonate3D: assignment of ionization states and hydrogen coordinates to macromolecular structures. Proteins 75:187–205. doi:10.1002/prot.22234
Halgren TA (1996) Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J Comput Chem 17:490–519. doi:10.1002/(SICI)1096-987X(199604)17:5/6<490:AID-JCC1>3.0.CO;2-P
Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE (2000) The protein data bank. Nucleic Acids Res 28:235–242. doi:10.1093/nar/28.1.235
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2791. doi:10.1002/jcc.21256
Trott O, Olson AJ (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461. doi:10.1002/jcc.21334
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47:1739–1749. doi:10.1021/jm0306430
Halgren TA, Murphy RB, Friesner RA, Beard HS, Frye LL, Pollard WT, Banks JL (2004) Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J Med Chem 47:1750–1759. doi:10.1021/jm030644s
Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD (2003) Improved protein-ligand docking using GOLD. Proteins 52:609–623. doi:10.1002/prot.10465
Chemical Computing Group Inc. (2015) Molecular Operating Environment (MOE), 2014.09, 1010 Sherbooke St. West, Suite #910, Montreal, QC, Canada, H3A 2R7
Korb O, Stützle T, Exner TE (2009) Empirical scoring functions for advanced protein-ligand docking with PLANTS. J Chem Inf Model 49:84–96. doi:10.1021/ci800298z
Ruiz-Carmona S, Alvarez-Garcia D, Foloppe N, Garmendia-Doval AB, Juhos S, Schmidtke P, Barril X, Hubbard RE, Morley SD (2014) rDock: a fast, versatile and open source program for docking ligands to proteins and nucleic acids. PLoS Comput Biol 10:e1003571. doi:10.1371/journal.pcbi.1003571
Corbeil CR, Williams CI, Labute P (2012) Variability in docking success rates due to dataset preparation. J Comput Aided Mol Des 26:775–786. doi:10.1007/s10822-012-9570-1
O’Boyle NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR (2011) Open Babel: an open chemical toolbox. J Cheminform 3:33. doi:10.1186/1758-2946-3-33
OpenEye Scientific Software Inc. (2016) OEChem. OpenEye Scientific Software Inc., Santa Fe
Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE (2004) UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem 25:1605–1612. doi:10.1002/jcc.20084
Davies M, Nowotka M, Papadatos G, Dedman N, Gaulton A, Atkinson F, Bellis L, Overington JP (2015) ChEMBL web services: streamlining access to drug discovery data and utilities. Nucleic Acids Res 43:W612–W620. doi:10.1093/nar/gkv352
Wang J, Wang W, Kollman PA, Case DA (2006) Automatic atom type and bond type perception in molecular mechanical calculations. J Mol Graph Model 25:247–260. doi:10.1016/j.jmgm.2005.12.005
Case D, Babin V, Berryman J, Betz R, Cai Q, Cerutti D, Cheatham Iii T, Darden T, Duke R, Gohlke H (2014) Amber14, version AMBER14; http://ambermd.org/ (accessed Oct 2015). University of California, San Francisco
Hornak V, Abel R, Okur A, Strockbine B, Roitberg A, Simmerling C (2006) Comparison of multiple Amber force fields and development of improved protein backbone parameters. Proteins 65:712–725. doi:10.1002/prot.21123
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25:1157–1174. doi:10.1002/jcc.20035
Bayly CI, Cieplak P, Cornell W, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97:10269–10280. doi:10.1021/j100142a004
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision B.01. http://gaussian.com/. Accessed Oct 2015. Gaussian, Inc.: Wallingford, CT
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103:8577. doi:10.1063/1.470117
Sabbadin D, Ciancetta A, Moro S (2014) Bridging molecular docking to membrane molecular dynamics to investigate GPCR-ligand recognition: the human A2A adenosine receptor as a key study. J Chem Inf Model 54:169–183. doi:10.1021/ci400532b
Williams T, Kelley C Gnuplot 4.5: an interactive plotting program, version 4.5; http://gnuplot.info (Accessed Oct 2015)
Zehnder L, Bennett M, Meng J, Huang B, Ninkovic S, Wang F, Braganza J, Tatlock J, Jewell T, Zhou JZ, Burke B, Wang J, Maegley K, Mehta PP, Yin MJ, Gajiwala KS, Hickey MJ, Yamazaki S, Smith E, Kang P, Sistla A, Dovalsantos E, Gehring MR, Kania R, Wythes M, Kung PP (2011) Optimization of potent, selective, and orally bioavailable pyrrolodinopyrimidine-containing inhibitors of heat shock protein 90. Identification of development candidate 2-amino-4-{4-chloro-2-[2-(4-fluoro-1H-pyrazol-1-yl)ethoxy]-6-methylphenyl}-N-(2,2-difluoropropyl)-5,7-dihydro-6H-pyrrolo[3,4-d]pyrimidine-6-carboxamide. J Med Chem 54:3368–3385. doi:10.1021/jm200128m
Roughley SD, Hubbard RE (2011) How well can fragments explore accessed chemical space? A case study from heat shock protein 90. J Med Chem 54:3989–4005. doi:10.1021/jm200350g
Miura T, Fukami TA, Hasegawa K, Ono N, Suda A, Shindo H, Yoon DO, Kim SJ, Na YJ, Aoki Y, Shimma N, Tsukuda T, Shiratori Y (2011) Lead generation of heat shock protein 90 inhibitors by a combination of fragment-based approach, virtual screening, and structure-based drug design. Bioorg Med Chem Lett 21:5778–5783. doi:10.1016/j.bmcl.2011.08.001
Congreve M, Chessari G, Tisi D, Woodhead AJ (2008) Recent developments in fragment-based drug discovery. J Med Chem 51:3661–3680. doi:10.1021/jm8000373
Bruncko M, Tahir SK, Song X, Chen J, Ding H, Huth JR, Jin S, Judge RA, Madar DJ, Park CH, Park CM, Petros AM, Tse C, Rosenberg SH, Elmore SW (2010) N-aryl-benzimidazolones as novel small molecule HSP90 inhibitors. Bioorg Med Chem Lett 20:7503–7506. doi:10.1016/j.bmcl.2010.10.010
Li J, Shi F, Xiong B, He J (2014) 4LWI, crystal structure of the human Hsp90-alpha N-domain bound to the hsp90 inhibitor FJ6. doi:10.2210/pdb4lwi/pdb
Brough PA, Barril X, Borgognoni J, Chene P, Davies NG, Davis B, Drysdale MJ, Dymock B, Eccles SA, Garcia-Echeverria C, Fromont C, Hayes A, Hubbard RE, Jordan AM, Jensen MR, Massey A, Merrett A, Padfield A, Parsons R, Radimerski T, Raynaud FI, Robertson A, Roughley SD, Schoepfer J, Simmonite H, Sharp SY, Surgenor A, Valenti M, Walls S, Webb P, Wood M, Workman P, Wright L (2009) Combining hit identification strategies: fragment-based and in silico approaches to orally active 2-aminothieno[2,3-d]pyrimidine inhibitors of the Hsp90 molecular chaperone. J Med Chem 52:4794–4809. doi:10.1021/jm900357y
Murray CW, Carr MG, Callaghan O, Chessari G, Congreve M, Cowan S, Coyle JE, Downham R, Figueroa E, Frederickson M, Graham B, McMenamin R, O’Brien MA, Patel S, Phillips TR, Williams G, Woodhead AJ, Woolford AJ (2010) Fragment-based drug discovery applied to Hsp90. Discovery of two lead series with high ligand efficiency. J Med Chem 53:5942–5955. doi:10.1021/jm100059d
Kang YN, Stuckey JA (2016) 4YKR, heat shock protein 90 bound to CS302. doi:10.2210/pdb4ykr/pdb
Kang YN, Stuckey JA (2016) 4YKY, heat shock protein 90 bound to CS319. doi:10.2210/pdb4yky/pdb
Crawford TD, Ndubaku CO, Chen H, Boggs JW, Bravo BJ, Delatorre K, Giannetti AM, Gould SE, Harris SF, Magnuson SR, McNamara E, Murray LJ, Nonomiya J, Sambrone A, Schmidt S, Smyczek T, Stanley M, Vitorino P, Wang L, West K, Wu P, Ye W (2014) Discovery of selective 4-Amino-pyridopyrimidine inhibitors of MAP4K4 using fragment-based lead identification and optimization. J Med Chem 57:3484–3493. doi:10.1021/jm500155b
Schröder P, Förster T, Kleine S, Becker C, Richters A, Ziegler S, Rauh D, Kumar K, Waldmann H (2015) Neuritogenic militarinone-inspired 4-hydroxypyridones target the stress pathway kinase MAP4K4. Angew Chem Int Ed Engl 54:12398–12403. doi:10.1002/anie.201501515
Wang L, Stanley M, Boggs JW, Crawford TD, Bravo BJ, Giannetti AM, Harris SF, Magnuson SR, Nonomiya J, Schmidt S, Wu P, Ye W, Gould SE, Murray LJ, Ndubaku CO, Chen H (2014) Fragment-based identification and optimization of a class of potent pyrrolo[2,1-f][1,2,4]triazine MAP4K4 inhibitors. Bioorg Med Chem Lett 24:4546–4552. doi:10.1016/j.bmcl.2014.07.071
Ndubaku CO, Crawford TD, Chen H, Boggs JW, Drobnick J, Harris SF, Jesudason R, McNamara E, Nonomiya J, Sambrone A, Schmidt S, Smyczek T, Vitorino P, Wang L, Wu P, Yeung S, Chen J, Chen K, Ding CZ, Wang T, Xu Z, Gould SE, Murray LJ, Ye W (2015) Structure-Based Design of GNE-495, a Potent and Selective MAP4K4 Inhibitor with Efficacy in Retinal Angiogenesis. ACS Med Chem Lett 6:913–918. doi:10.1021/acsmedchemlett.5b00174
Acknowledgments
The computational work coordinated by S.M. has been supported with financial support from the University of Padova, Italy. MMS lab is also very grateful to Chemical Computing Group, OpenEye and Acellera for the scientific and technical partnership. S.M. participates in the European COST Action CM1207 (GLISTEN). The work of M.S. has been supported by University of Padova, Italy (UNIPD, Progetto Giovani Studiosi 2013: Protocol number 79122). Finally, MMS lab is extremely grateful to the organizers of the D3R Grand Challenge for the perfect organization of the event.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Veronica Salmaso and Mattia Sturlese have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Salmaso, V., Sturlese, M., Cuzzolin, A. et al. DockBench as docking selector tool: the lesson learned from D3R Grand Challenge 2015. J Comput Aided Mol Des 30, 773–789 (2016). https://doi.org/10.1007/s10822-016-9966-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-016-9966-4