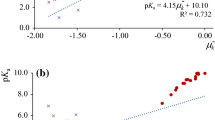
Abstract
We describe a novel approach of reaction representation as a combination of two mixtures: a mixture of reactants and a mixture of products. In turn, each mixture can be encoded using an earlier reported approach involving simplex descriptors (SiRMS). The feature vector representing these two mixtures results from either concatenated product and reactant descriptors or the difference between descriptors of products and reactants. This reaction representation doesn’t need an explicit labeling of a reaction center. The rigorous “product-out” cross-validation (CV) strategy has been suggested. Unlike the naïve “reaction-out” CV approach based on a random selection of items, the proposed one provides with more realistic estimation of prediction accuracy for reactions resulting in novel products. The new methodology has been applied to model rate constants of E2 reactions. It has been demonstrated that the use of the fragment control domain applicability approach significantly increases prediction accuracy of the models. The models obtained with new “mixture” approach performed better than those required either explicit (Condensed Graph of Reaction) or implicit (reaction fingerprints) reaction center labeling.







Similar content being viewed by others
References
Chen WL, Chen DZ, Taylor KT (2013) Automatic reaction mapping and reaction center detection. Wiley Interdiscip Rev Comput Mol Sci 3(6):560–593. doi:10.1002/wcms.1140
Zhang J, Kleinöder T, Gasteiger J (2006) Prediction of pKa values for aliphatic carboxylic acids and alcohols with empirical atomic charge descriptors. J Chem Inf Model 46(6):2256–2266. doi:10.1021/ci060129d
Gasteiger J, Hondelmann U, Rose P, Witzenbichler W (1995) Computer-assisted prediction of the degradation of chemicals: hydrolysis of amides and benzoylphenylureas. J Chem Soc Perkin Trans 2(2):193–204. doi:10.1039/p29950000193
Varnek A, Fourches D, Horvath D, Klimchuk O, Gaudin C, Vayer P, Solov’ev V, Hoonakker F, Tetko IV, Marcou G (2008) ISIDA—platform for virtual screening based on fragment and pharmacophoric descriptors. Curr Comput Aided Drug Des 4(3):191–198. doi:10.2174/157340908785747465
Ruggiu F, Marcou G, Varnek A, Horvath D (2010) ISIDA property-labelled fragment descriptors. Mol Inform 29(12):855–868. doi:10.1002/minf.201000099
Varnek A, Fourches D, Hoonakker F, Solov’ev VP (2005) Substructural fragments: an universal language to encode reactions, molecular and supramolecular structures. J Comput Aided Mol Des 19(9):693–703. doi:10.1007/s10822-005-9008-0
Hoonakker F, Lachiche N, Varnek A, Wagner A (2011) A representation to apply usual data mining techniques to chemical reactions—illustration on the rate constant of SN2 reactions in water. Int J Artif Intell Tools 20(02):253–270. doi:10.1142/S0218213011000140
de Luca A, Horvath D, Marcou G, Solov’ev V, Varnek A (2012) Mining chemical reactions using neighborhood behavior and condensed graphs of reactions approaches. J Chem Inf Model 52(9):2325–2338. doi:10.1021/ci300149n
Madzhidov TI, Polishchuk PG, Nugmanov RI, Bodrov AV, Lin AI, Baskin II, Varnek AA, Antipin IS (2014) Structure-reactivity relationships in terms of the condensed graphs of reactions. Russ J Org Chem 50(4):459–463. doi:10.1134/S1070428014040010
Nugmanov RI, Madzhidov TI, Haliullina GR, Baskin II, Antipin IS, Varnek A (2014) Development of “structure-reactivity” models for nucleophilic substitution reactions with participation of azides. J Struct Chem 55(6):1080–1087
Madzhidov T, Bodrov A, Gimadiev T, Nugmanov R, Antipin I, Varnek A (2015) Obtaining structure-reactivity relationships for bimolecular elimination reactions with Condensed Reaction Graph approach. J Struct Chem 56(7):1227–1234
Marcou G, Aires de Sousa J, Latino DARS, de Luca A, Horvath D, Rietsch V, Varnek A (2015) Expert system for predicting reaction conditions: the michael reaction case. J Chem Inf Model 55(2):239–250. doi:10.1021/ci500698a
Faulon J-L, Visco DP, Pophale RS (2003) The signature molecular descriptor. 1. Using extended valence sequences in QSAR and QSPR studies. J Chem Inf Comput Sci 43(3):707–720. doi:10.1021/ci020345w
Ridder L, Wagener M (2008) SyGMa: combining expert knowledge and empirical scoring in the prediction of metabolites. ChemMedChem 3(5):821–832. doi:10.1002/cmdc.200700312
Schneider N, Lowe DM, Sayle RA, Landrum GA (2015) Development of a novel fingerprint for chemical reactions and its application to large-scale reaction classification and similarity. J Chem Inf Model 55(1):39–53. doi:10.1021/ci5006614
Zhang Q-Y, Aires-de-Sousa J (2005) Structure-based classification of chemical reactions without assignment of reaction centers. J Chem Inf Model 45(6):1775–1783. doi:10.1021/ci0502707
Kravtsov AA, Karpov PV, Baskin II, Palyulin VA, Zefirov NS (2011) Prediction of rate constants of SN2 reactions by the multicomponent QSPR method. Dokl Chem 440 (2):299–301. doi:10.1134/s0012500811100107
Faulon J-L, Misra M, Martin S, Sale K, Sapra R (2008) Genome scale enzyme—metabolite and drug—target interaction predictions using the signature molecular descriptor. Bioinformatics 24(2):225–233. doi:10.1093/bioinformatics/btm580
Kravtsov AA, Karpov PV, Baskin II, Palyulin VA, Zefirov NS (2011) Prediction of the preferable mechanism of nucleophilic substitution at saturated carbon atom and prognosis of S N 1 rate constants by means of QSPR. Dokl Chem 441 (1):314–317. doi:10.1134/s0012500811110048
Muller C, Marcou G, Horvath D, Aires-de-Sousa J, Varnek A (2012) Models for identification of erroneous atom-to-atom mapping of reactions performed by automated algorithms. J Chem Inf Model 52(12):3116–3122. doi:10.1021/ci300418q
Patel H, Bodkin MJ, Chen B, Gillet VJ (2009) Knowledge-based approach to de novo design using reaction vectors. J Chem Inf Model 49(5):1163–1184. doi:10.1021/ci800413m
Oprisiu I, Varlamova E, Muratov E, Artemenko A, Marcou G, Polishchuk P, Kuz’min V, Varnek A (2012) QSPR approach to predict nonadditive properties of mixtures. Application to bubble point temperatures of binary mixtures of liquids. Mol Inform 31(6–7):491–502. doi:10.1002/minf.201200006
Palm VA (1974–1978) Tables of rate and equilibrium constants of heterolytic organic reactions, vol 1–5. Moscow
Catalán J, Díaz C (1997) A generalized solvent acidity scale: the solvatochromism of o-tert-butylstilbazolium betaine dye and its homomorph o, o′-di-tert-butylstilbazolium betaine dye. Liebigs Ann 1997 (9):1941–1949. doi:10.1002/jlac.199719970921
Catalán J, Díaz C, López V, Pérez P, De Paz J-LG, Rodríguez JG (1996) A generalized solvent basicity scale: the solvatochromism of 5-nitroindoline and its homomorph 1-methyl-5-nitroindoline. Liebigs Ann 1996 (11):1785–1794. doi:10.1002/jlac.199619961112
Catalán J, López V, Pérez P, Martin-Villamil R, Rodríguez J-G (1995) Progress towards a generalized solvent polarity scale: The solvatochromism of 2-(dimethylamino)-7-nitrofluorene and its homomorph 2-fluoro-7-nitrofluorene. Liebigs Ann 1995 (2):241–252. doi:10.1002/jlac.199519950234
Taft RW, Kamlet MJ (1976) The solvatochromic comparison method. 2. The .alpha.-scale of solvent hydrogen-bond donor (HBD) acidities. J Am Chem Soc 98(10):2886–2894. doi:10.1021/ja00426a036
Kamlet MJ, Taft RW (1976) The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J Am Chem Soc 98(2):377–383. doi:10.1021/ja00418a009
Kamlet MJ, Abboud JL, Taft RW (1977) The solvatochromic comparison method. 6. The .pi.* scale of solvent polarities. J Am Chem Soc 99(18):6027–6038. doi:10.1021/ja00460a031
cxcalc. 5.4 edn. Chemaxon, Budapest, Hungary
Kuz’min VE, Artemenko AG, Muratov EN (2008) Hierarchical QSAR technology based on the Simplex representation of molecular structure. J Comput Aided Mol Des 22(6–7):403–421. doi:10.1007/s10822-008-9179-6
Kuz’min VE, Artemenko AG, Polischuk PG, Muratov EN, Khromov AI, Liahovskiy AV, Andronati SA, Makan SY (2005) Hierarchic system of QSAR models (1D-4D) on the base of simplex representation of molecular structure. J Mol Model 11:457–467. doi:10.1007/s00894-005-0237-x
RDKit: Open-Source Cheminformatics. http://www.rdkit.org
Carhart RE, Smith DH, Venkataraghavan R (1985) Atom pairs as molecular features in structure-activity studies: definition and applications. J Chem Inf Comput Sci 25(2):64–73. doi:10.1021/ci00046a002
Rogers D, Hahn M (2010) Extended-Connectivity Fingerprints. J Chem Inf Model 50(5):742–754. doi:10.1021/ci100050t
Nilakantan R, Bauman N, Dixon JS, Venkataraghavan R (1987) Topological torsion: a new molecular descriptor for SAR applications. Comparison with other descriptors. J Chem Inf Comput Sci 27(2):82–85. doi:10.1021/ci00054a008
Liaw A, Wiener M (2002) Classification and regression by randomForest. R News 2(3):18–22
Max Kuhn. Contributions from Jed Wing and Steve Weston and Andre Williams and Chris Keefer and Allan Engelhardt and Tony Cooper and Zachary Mayer and the R Core Team caret: Classification and Regression Training (2014). R package version 6.0–30 edn.
Acknowledgements
This work was supported by Russian Science Foundation, Grant No. 14-43-00024.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Polishchuk, P., Madzhidov, T., Gimadiev, T. et al. Structure–reactivity modeling using mixture-based representation of chemical reactions. J Comput Aided Mol Des 31, 829–839 (2017). https://doi.org/10.1007/s10822-017-0044-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-017-0044-3