Abstract
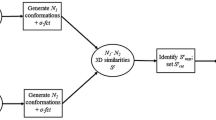
Shape similarity searching is a popular approach for ligand-based virtual screening on the basis of three-dimensional reference compounds. It is generally thought that well-defined experimentally determined binding modes of active reference compounds provide the best possible basis for shape searching. Herein, we show that experimental binding modes are not essential for successful shape similarity searching. Furthermore, we show that ensembles of analogs of X-ray ligands—in the absence of these ligands—further improve the search performance of single crystallographic reference compounds. This is even the case if ensembles of virtually generated analogs are used whose activity status is unknown. Taken together, the results of our study indicate that analog ensembles representing fuzzy reference states are effective starting points for shape similarity searching.




Similar content being viewed by others
References
Varnek A, Tropsha A (2008) Chemoinformatics approaches to virtual screening. Royal Society of Chemistry, Cambridge
Geppert H, Vogt M, Bajorath J (2010) Current trends in ligand-based virtual screening: molecular representations, data mining methods, new application areas, and performance evaluation. J Chem Inf Model 50:205–216
Naylor E, Arredouani A, Vasudevan SR, Lewis AM, Parkesh R, Mizote A, Rosen D, Thomas JM, Izumi M, Ganesan A, Galione A, Churchill GC (2009) Identification of a chemical probe for NAADP by virtual screening. Nat Chem Biol 5:220–222
Rush TS, Grant JA, Mosyak L, Nicholls A (2005) A shape-based 3-D scaffold hopping method and its application to a bacterial protein–protein interaction. J Med Chem 48:1489–1495
Hawkins PCD, Skillman AG, Nicholls A (2007) Comparison of shape-matching and docking as virtual screening tools. J Med Chem 50:74–82
Myrianthopoulos V, Gaboriaud-Kolar N, Tallant C, Hall M-L, Grigoriou S, Brownlee PM, Fedorov O, Rogers C, Heidenreich D, Wanior M, Drosos N, Mexia N, Savitsky P, Bagratuni T, Kastritis E, Terpos E, Filippakopoulos P, Müller S, Skaltsounis AL, Downs JA, Knapp S, Mikros E (2016) Discovery and optimization of a selective ligand for the switch/sucrose nonfermenting-related bromodomains of polybromo protein-1 by the use of virtual screening and hydration analysis. J Med Chem 59:8787–8803
Kaserer T, Rigo R, Schuster P, Alcaro S, Sissi C, Schuster D (2016) Optimized virtual screening workflow for the identification of novel g-quadruplex ligands. J Chem Inf Model 56:484–500
Cappel D, Dixon SL, Sherman W, Duan J (2015) Exploring conformational search protocols for ligand-based virtual screening and 3-D QSAR modeling. J Comput Aided Mol Des 29:165–182
Kirchmair J, Distinto S, Markt P et al (2009) How to optimize shape-based virtual screening: choosing the right query and including chemical information. J Chem Inf Model 49:678–692
Anighoro A, Bajorath J (2017) Compound ranking based on fuzzy three-dimensional similarity improves the performance of docking into homology models of G-protein-coupled receptors. ACS Omega 2:2583–2592
Hert J, Willett P, Wilton DJ et al (2005) Enhancing the effectiveness of similarity-based virtual screening using nearest-neighbor information. J Med Chem 48:7049–7054
Willett P (2006) Similarity-based virtual screening using 2D fingerprints. Drug Discov Today 11:1046–1053
Yu X, Geer LY, Han L, Bryant SH (2015) Target enhanced 2D similarity search by using explicit biological activity annotations and profiles. J Cheminform 7:55
Hu Y, Furtmann N, Bajorath J (2015) Extension of three-dimensional activity cliff information through systematic mapping of active analogs. RSC Adv 5:43006–43015
Furtmann N, Hu Y, Bajorath J (2015) Comprehensive analysis of three-dimensional activity cliffs formed by kinase inhibitors with different binding modes and cliff mapping of structural analogues. J Med Chem 58:252–264
Weber J, Achenbach J, Moser D, Proschak E (2013) VAMMPIRE: a matched molecular pairs database for structure-based drug design and optimization. J Med Chem 56:5203–5207
Furtmann N, Hu Y, Gütschow M, Bajorath J (2015) Identification of interaction hot spots in structures of drug targets on the basis of three-dimensional activity cliff information. Chem Biol Drug Des 86:1458–1465
Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, Light Y, McGlinchey S, Michalovich D, Al-Lazikani B et al (2012) ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic Acids Res 40:D1100–D1107
Hu X, Hu Y, Vogt M, Stumpfe D, Bajorath J (2012) MMP-cliffs: systematic identification of activity cliffs on the basis of matched molecular pairs. J Chem Inf Model 52:1138–1145
Hussain J, Rea C (2010) Computationally efficient algorithm to identify matched molecular pairs (MMPs) in large data sets. J Chem Inf Model 50:339–348
Kunimoto R, Miyao T, Bajorath J (2018) Computational method for estimating progression saturation of analog series. RSC Adv 8:5484–5492
OEOmega TK version 2.6.7; OpenEye Scientific Software, Santa Fe, NM
OEFF TK version 2.0.1; OpenEye Scientific Software, Santa Fe, NM
ROCS version 3.2.2.2, OpenEye Scientific Software, Santa Fe, NM
Mysinger MM, Carchia M, Irwin JJ, Shoichet BK (2012) Directory of useful decoys, enhanced (DUD-E): better ligands and decoys for better benchmarking. J Med Chem 55:6582–6594
Rogers D, Hahn M (2010) Extended-connectivity fingerprints. J Chem Inf Model 50:742–754
Gardiner EJ, Gillet VJ, Haranczyk M et al (2009) Turbo similarity searching: effect of fingerprint and dataset on virtual-screening performance. Stat Anal Data Min 2:103–114
Acknowledgements
We thank the OpenEye Scientific Software, Inc., for providing a free academic license of the OpenEye chemistry toolkit and the ROCS program.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Miyao, T., Bajorath, J. Exploring ensembles of bioactive or virtual analogs of X-ray ligands for shape similarity searching. J Comput Aided Mol Des 32, 759–767 (2018). https://doi.org/10.1007/s10822-018-0128-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-018-0128-8