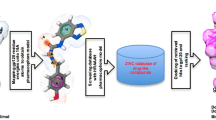
Abstract
Attachment of envelope glycoprotein gp120 to the host cell receptor CD4 is the first step during the human immunodeficiency virus-1 (HIV-1) entry into the host cells that makes it a promising target for drug design. To elucidate the crucial three dimensional (3D) structural features of reported HIV-1 gp120 CD4 binding inhibitors, 3D pharmacophores were generated and receptor based approach was employed to quantify these structural features. A four-partial least square factor model with good statistics and predictive ability was generated for the dataset of 100 molecules. To further ascertain the structural requirement for gp120-CD4 binding inhibition, molecular interaction studies of inhibitors with gp120 was carried out by performing molecular docking using Glide 5.6. Based on these studies, structural requirements were drawn and new molecules were designed accordingly to yield new sulphonamides derivatives. A water based green synthetic approach was adopted to obtain these compounds which were evaluated for their HIV-1 gp120 CD4 binding inhibition. The newly synthesized compounds exhibited remarkable activity (10-fold increase) when compared with the standard BMS 806. Further the stability of newly synthesized derivatives with HIV-1 gp120 was also investigated through molecular dynamics simulation studies. This provides a proof of concept for molecular modeling based design of new inhibitors for inhibition of HIV-1 gp120 CD4 interaction.












Similar content being viewed by others
References
Taiwo B, Hicks C, Eron JJ (2010) Unmet therapeutic needs in the new era of combination antiretroviral therapy for HIV-1. J Antimicrob Chemother 65(6):1100–1107. https://doi.org/10.1093/jac/dkq096
Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (1998) Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393(6686):648–659.
Kwong PD, Wyatt R, Majeed S, Robinson J, Sweet RW, Sodroski J, Hendrickson WA (2000) Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure 8(12):1329–1339. https://doi.org/10.1016/S0969-2126(00)00547-5
Wyatt R, Kwong PD, Desjardins E, Sweet RW, Robinson J, Hendrickson WA, Sodroski JG (1998) The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393(6686):705–711.
Vermeire K, Schols D (2005) Anti-HIV agents targeting the interaction of gp120 with the cellular CD4 receptor. Expert Opin Investig Drugs 14(10):1199–1212.
Kadow J, Wang HG, Lin PF (2006) Small molecule HIV1 gp120 inhibitors to prevent HIV-1 entry: an emerging opportunity for drug development. Curr Opin Investig Drugs 7(8):721–726
Li W, Lu L, Li W, Jiang S (2017) Small-molecule HIV-1 entry inhibitors targeting gp120 and gp41: a patent review (2010–2015). Expert Opin Ther Pat 27(6):707–719. https://doi.org/10.1080/13543776.2017.1281249
Curreli F, Kwon YD, Zhang H, Scacalossi D, Belov DS, Tikhonov AA, Andreev IA, Altieri A, Kurkin AV, Kwong PD, Debnath AK (2015) Structure-based design of a small molecule CD4-antagonist with broad spectrum anti-HIV-1 activity. J Med Chem 58(17):6909–6927. https://pubs.acs.org/doi/10.1021/acs.jmedchem.5b00709
Herschhorn A, Gu C, Espy N, Richard J, Finzi A, Sodroski JG (2014) A broad HIV-1 inhibitor blocks envelope glycoprotein transitions critical for entry. Nat Chem Biol 10(10):845–852.
Courter JR, Madani N, Sodroski J, Schön A, Freire E, Kwong PD, Hendrickson WA, Chaiken IM, LaLonde JM, Smith III AB (2014) Structure-based design, synthesis and validation of CD4-mimetic small molecule inhibitors of HIV-1 entry: conversion of a viral entry agonist to an antagonist. Acc Chem Res 47(4):1228–1237.
Lin PF, Blair W, Wang T, Spicer T, Guo Q, Zhou N, Gong YF, Wang HG, Rose R, Yamanaka G, Robinson B, Li CB, Fridell R, Deminie C, Demers G, Yang Z, Zadjura L, Meanwell N, Colonno R (2003) A small molecule HIV-1 inhibitor that targets the HIV-1 envelope and inhibits CD4 receptor binding. Proc Natl Acad Sci USA 100(19):11013–11018. https://doi.org/10.1073/pnas.1832214100
Si Z, Madani N, Cox JM, Chruma JJ, Klein JC, Schön A, Phan N, Wang L, Biorn AC, Cocklin S, Chaiken I, Freire E, Smith AB III, Sodroski JG (2004) Small-molecule inhibitors of HIV-1 entry block receptor-induced conformational changes in the viral envelope glycoproteins. Proc Natl Acad Sci USA 101(14):5036–5041. https://doi.org/10.1073/pnas.0307953101
Ho HT, Fan L, Nowicka-Sans B, McAuliffe B, Li CB, Yamanaka G, Zhou N, Fang H, Dicker I, Dalterio R, Gong YF, Wang T, Yin Z, Ueda Y, Matiskella J, Kadow J, Clapham P, Robinson J, Colonno R, Lin PF (2006) Envelope conformational changes induced by human immunodeficiency virus type 1 attachment inhibitors prevent CD4 binding and downstream entry events. J Virol 80(8):4017–4025.
Xie H, Danny DN, Savinov SN, Dey B, Kwong PD, Wyatt R, Smith AB, Hendrickson WA (2007) Structure–activity relationships in the binding of chemically derivatized CD4 to gp120 from human immunodeficiency virus. J Med Chem 50(20):4898–4908. https://doi.org/10.1021/jm070564e
Caporuscio F, Tafi A, Gonzalez E, Manetti F, Este JA, Botta M (2009) A dynamic target-based pharmacophoric model mapping the CD4 binding site on HIV-1 gp120 to identify new inhibitors of gp120-CD4 protein–protein interactions. Bioorg Med Chem Lett 19(21):6087–6091. https://doi.org/10.1016/j.bmcl.2009.09.029
Yamada Y, Ochiai C, Yoshimura K, Tanaka T, Ohashi N, Narumi T, Nomura W, Harada S, Matsushita S, Tamamura H (2010) CD4 mimics targeting the mechanism of HIV entry. Bioorg Med Chem Lett 20(1):354–358. https://doi.org/10.1016/j.bmcl.2009.10.098
Meanwell NA, Wallace OB, Fang H, Wang H, Deshpande M, Wang T, Yin Z, Zhang Z, Pearce BC, James J, Yeung KS, Qiu Z, Wright JJK, Yang Z, Zadjura L, Tweedie DL, Yeola S, Zhao F, Ranadive S, Robinson BA, Gong YF, Wang HG, Spicer TP, Blair WS, Shi PY, Colono RJ, Lin PF (2009) Inhibitors of HIV-1 attachment. Part 2: an initial survey of indole substitution patterns. Bioorg Med Chem Lett 19(7):1977–1981. https://doi.org/10.1016/j.bmcl.2009.02.040
Meanwell NA, Wallace OB, Wang H, Deshpande M, Pearce BC, Trehan A, Yeung KS, Qiu Z, Wright JJ, Robinson BA, Gong YF, Wang HG, Spicer TP, Blair WS, Shi PY, Lin PF (2009) Inhibitors of HIV-1 attachment. Part 3: a preliminary survey of the effect of structural variation of the benzamide moiety on antiviral activity. Bioorg Med Chem Lett 19(17):5136–5139. https://doi.org/10.1016/j.bmcl.2009.07.027
Wang T, Kadow JF, Zhang Z, Yin Z, Gao Q, Wu D, Parker DD, Yang Z, Zadjura L, Robinson BA, Gong YF, Blair WS, Shi PY, Yamanaka G, Lin PF, Meanwell NA (2009) Inhibitors of HIV-1 attachment. Part 4: a study of the effect of piperazine substitution patterns on antiviral potency in the context of indole-based derivatives. Bioorg Med Chem Lett 19(17):5140–5145. https://doi.org/10.1016/j.bmcl.2009.07.076
Wang T, Yin Z, Zhang Z, Bender JA, Yang Z, Johnson G, Yang Z, Zadjura LM, D’Arienzo CJ, Parker DD, Gesenberg C, Yamanaka GA, Gong YF, Ho HT, Fang H, Zhou N, McAuliffe BV, Eggers BJ, Fan L, Nowicka-Sans B, Dicker IB, Gao Q, Colonno RJ, Lin PF, Meanwell NA, Kadow JF (2009) Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment. 5. An evolution from indole to azaindoles leading to the discovery of 1-(4-benzoylpiperazin-1-yl)-2-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)ethane-1,2-dione (BMS-488043), a drug candidate that demonstrates antiviral activity in HIV-1-infected subjects. J Med Chem 52(23):7778–7787. https://doi.org/10.1021/jm900843g
Yeung KS, Qiu Z, Xue Q, Fang H, Yang Z, Zadjura L, D’Arienzo CJ, Eggers BJ, Riccardi K, Shi PY, Gong YF, Browning MR, Gao Q, Hansel S, Santone K, Lin PF, Meanwell NA, Kadow JF (2013) Inhibitors of HIV-1 attachment. Part 7: indole-7-carboxamides as potent and orally bioavailable antiviral agents. Bioorg Med Chem Lett 23(1):198–202. https://doi.org/10.1016/j.bmcl.2012.10.115
Yeung KS, Qiu Z, Yin Z, Trehan A, Fang H, Pearce B, Yang Z, Zadjura L, D’Arienzo CJ, Riccardi K, Shi PY, Spicer TP, Gong YF, Browning MR, Hansel S, Santone K, Barker J, Coulter T, Lin PF, Meanwell NA, Kadow JF (2013) Inhibitors of HIV-1 attachment. Part 8: the effect of C7-heteroaryl substitution on the potency, and in vitro and in vivo profiles of indole-based inhibitors. Bioorg Med Chem Lett 23(1):203–208. https://doi.org/10.1016/j.bmcl.2012.10.117
Kadow JF, Ueda Y, Meanwell NA, Connolly TP, Wang T, Chen CP, Yeung KS, Zhu J, Bender JA, Yang Z, Parker D, Lin PF, Colonno RJ, Mathew M, Morgan D, Zheng M, Chien C, Grasela D (2012) Inhibitors of human immunodeficiency virus type 1 (HIV-1) attachment 6. Preclinical and human pharmacokinetic profiling of BMS-663749, a phosphonooxymethyl prodrug of the HIV-1 attachment inhibitor 2-(4-benzoyl-1-piperazinyl)-1-(4,7-dimethoxy-1H-pyrrolo[2,3-c]pyridin-3-yl)-2-oxoethanone (BMS-488043). J Med Chem 55(5):2048–2056. https://doi.org/10.1021/jm201218m
Wang T, Yang Z, Zhang Z, Gong YF, Riccardi KA, Lin PF, Parker DD, Rahematpura S, Mathew M, Zheng M, Meanwell NA, Kadow JF, Bender JA (2013) Inhibitors of HIV-1 attachment. Part 10. The discovery and structure–activity relationships of 4-azaindole cores. Bioorg Med Chem Lett 23(1):213–217. https://doi.org/10.1016/j.bmcl.2012.10.120
Bender JA, Yang Z, Eggers B, Gong YF, Lin PF, Parker DD, Rahematpura S, Zheng M, Meanwell NA, Kadow JF (2013) Inhibitors of HIV-1 attachment. Part 11: the discovery and structure–activity relationships associated with 4,6-diazaindole cores. Bioorg Med Chem Lett 23(1):218–222. https://doi.org/10.1016/j.bmcl.2012.10.118
Ligprep 2.0 (2010) Schrӧdinger, LLC, New York
Kaminski GA, Friesner RA, Tirado-Rives J, Jorgensen WL (2001) Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J Phys Chem B 105(28):6474–6487. https://doi.org/10.1021/jp003919d
PHASE 3.0 (2010) Schrӧdinger, LLC, New York
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening-1. Methodology and preliminary results. J Comput Aided Mol Des 20(10–11):647–671. https://doi.org/10.1007/s10822-006-9087-6
Dixon SL, Smondyrev AM, Rao S (2006) PHASE: a new approach to pharmacophore modeling and 3D database searching. Chem Biol Drug Des 67(5):370–372. https://doi.org/10.1111/j.1747-0285.2006.00384.x
Teli MK, Rajanikant GK (2011) Pharmacophore generation and atom-based 3D-QSAR of novel quinoline-3-carbonitrile derivatives as Tpl2 kinase inhibitors. J Enzyme Inhib Med Chem 27(4):558–570. https://doi.org/10.3109/14756366.2011.603128
Mahipal, Tanwar OP, Karthikeyan C, Moorthy NS, Trivedi P (2010) 3D QSAR of aminophenyl benzamide derivatives as histone deacetylase inhibitors. Med Chem 6(5):277–285.
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH, Shelley M, Perry JK, Shaw DE, Francis P, Shenkin PS (2004) Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J Med Chem 47(7):1739–1749. https://doi.org/10.1021/jm0306430
Lyne PD, Lamb ML, Saeh JC (2006) Accurate prediction of the relative potencies of members of a series of kinase inhibitors using molecular docking and MM-GBSA scoring. J Med Chem 49(16):4805-4808. https://pubs.acs.org/doi/10.1021/jm060522a
Desmond 3.8 (2014) Shaw Research DE, New York
Peddi SR, Sivan SK, Manga V (2016) An integrated molecular modeling approach for in silico design of new tetracyclic derivatives as ALK inhibitors. J Recept Signal Transduct Res 36(5):488–504. https://doi.org/10.3109/10799893.2015.1130057
Sivan SK, Vangala R, Manga V (2013) Molecular docking guided structure based design of symmetrical N, N′-disubstituted urea/thiourea as HIV-1 gp120-CD4 binding inhibitors. Bioorg Med Chem 21(15):4591–4599. https://doi.org/10.1016/j.bmc.2013.05.038
Lu RJ, John AT, Tatiana Z, Olga K, Vitalii K, Svetlana K, Sergey S, Jason P, Sagun T, Enugurthi B, Yang Y, Jian W, Stephanie F, Shelly F, Alana S, Jiying Z, Sherry SO, Michael G, Dani B, Brian B, Barney K, Peter J, Alisher K, Ma YA, Cynthia J, Changhui L, Tatiana P, Tong Z, Alexander C, Li R, Connie S (2007) Design and synthesis of human immunodeficiency virus entry inhibitors: sulfonamide as an isostere for the α-ketoamide group. J Med Chem 50(26):6535–6544. https://doi.org/10.1021/jm070650e
Hansch C, Sammes PG, Taylor JB (1990) Comprehensive medicinal chemistry. Pergamon Press, New York
Yoshino H, Ueda N, Nijima J, Sugumi H, Kotake Y, Koyanagi N, Yoshimatsu NK, Asada M, Watanabe T, Nagasu T, Tsukahara K, Iijima A, Kitoh K (1992) New sulfonamides as potential, systemically active antitumor agents. J Med Chem 35(13):2496–2497. https://doi.org/10.1021/jm00091a018
De Clercq E (2001) New developments in anti-HIV chemotherapy. Curr Med Chem 8(13):1543–1572.
Rotella DP (2002) Phosphodiesterase 5 inhibitors: current status and potential applications. Nat Rev Drug Discov 1(9):674–682. https://doi.org/10.1038/nrd893
Alonso DA, Andersson PG (1998) Deprotection of sulfonyl aziridines. J Org Chem 63(25):9455–9461. https://doi.org/10.1021/jo9815296
Pak CS, Lim DS (2001) Deprotection of 2-pyridyl sulfonyl group from pyridine-2-sulfonamides by magnesium in methanol. Synth Commun 31(14):2209–2214. https://doi.org/10.1081/SCC-100104475
Kamal A, Chouhan G (2004) Chemoenzymatic synthesis of enantiomerically pure 1,2-diols employing immobilized lipase in the ionic liquid [bmim]PF6. Tetrahedron Lett 45(48):8801–8805. https://doi.org/10.1016/j.tetlet.2004.10.015
Kamal A, Chouhan G (2005) A task-specific ionic liquid [bmim]SCN for the conversion of alkyl halides to alkyl thiocyanates at room temperature. Tetrahedron Lett 46(9):1489–1491. https://doi.org/10.1016/j.tetlet.2005.01.040
Kamal A, Chouhan G (2005) Chemoenzymatic synthesis of calcilytic agent NPS-2143 employing a lipase-mediated resolution protocol. Tetrahedron Asymmetry 16(16):2784–2789. https://doi.org/10.1016/j.tetasy.2005.07.022
Kamal A, Reddy DR, Rajendar (2007) Direct one-pot synthesis of β-hydroxysulfides from terminal olefins in a mixture of [bmim][BF4] and water in presence of molecular oxygen. J Mol Catal A 272(1–2):26–30
Kamal A, Reddy DR, Rajendar (2006) Polyethylene glycol (PEG) as an efficient recyclable medium for the synthesis of β-amino sulfides. Tetrahedron Lett 47(13):2261–2264. https://doi.org/10.1016/j.tetlet.2006.01.086
Kamal A, Shankaraiah N, Reddy KL, Devaiah V (2006) Selective reduction of aromatic azides in solution/solid-phase and resin cleavage by employing BF3 OEt2/EtSH. Preparation of DC-81. Tetrahedron Lett 47(25):4253–4257. https://doi.org/10.1016/j.tetlet.2006.04.025
Kamal A, Shankaraiah N, Reddy KL, Devaiah V (2006) An efficient solid-phase synthesis of biologically important DNA-interactive pyrrolo[2,1-c][1,4]benzodiazepine dimers (DSB-120) and their C2-fluorinated analogues. Tetrahedron Lett 47(37):6553–6556. https://doi.org/10.1016/j.tetlet.2006.07.016
Kamal A, Reddy JS, Bharathi EV, Dastagiri D (2008) Base-free monosulfonylation of amines using tosyl or mesyl chloride in water. Tetrahedron Lett 49(2):348–353. https://doi.org/10.1016/j.tetlet.2007.11.044
Wu HY, Li H, Zhu BL, Wang SR, Zhang SM, Wu SH, Huang WP (2008) The synthesis and crystal structures of new 2-aminomethylbenzimidazole zinc(II) complexes exhibiting luminescence. Transit Met Chem 33(1):9–15. https://doi.org/10.1007/s11243-007-9007-1
Furniss BS, Hannafor AJ, Rogers V, Smith PWG, Tatchell AR (2004) Vogel’s text book of practical organic chemistry. Longman, New York
Acknowledgements
We greatly acknowledge Schrӧdinger LLC, New York for providing the software. This research was made possible through grants from DST-SERB (EEQ/2018/000117).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors do not have any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vangala, R., Sivan, S.K., Peddi, S.R. et al. Computational design, synthesis and evaluation of new sulphonamide derivatives targeting HIV-1 gp120. J Comput Aided Mol Des 34, 39–54 (2020). https://doi.org/10.1007/s10822-019-00258-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-019-00258-0