Abstract
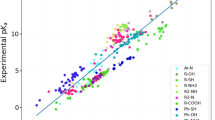
Accurate solvation free energy ΔGsolv predictions require well parametrized force fields. In order to refit Lennard-Jones (LJ) parameters for improved ΔGsolv predictions for a variety of compound classes and chemical environments, a large number of ΔGsolv calculations is required. As the calculation of ΔGsolv is computational expensive, there is need for efficient but precise calculation methods. In this work, we focus on the computation of non-aqueous solvation free energies. We compare ΔGsolv results from highly precise reference simulations for transferring a solute from the vacuum into a condensed phase and results obtained from a thermodynamic cycle implementation. As test systems, we alter LJ parameters ε and σ of widely used GAFF atom types ca, cl, n1, oh and os in various solute/solvent combinations. We examine the degree of configurational space overlap and find an impact by hydrogen bonds and the solvent accessible surface area. We conclude that the application of a thermodynamic cycle for the parametrization of force fields targeting ΔGsolv is useful if the adaptation of LJ parameters is limited to atom types in the solute or if only ε is changed.








Similar content being viewed by others
References
Hansen N, van Gunsteren WF (2014) Practical aspects of free-energy calculations: a review. J Chem Theory Comput 10(7):2632–2647. https://doi.org/10.1021/ct500161f
Klimovich PV, Shirts MR, Mobley DL (2015) Guidelines for the analysis of free energy calculations. J Comput Aided Mol Des 29(5):397–411. https://doi.org/10.1007/s10822-015-9840-9
Bruckner S, Boresch S (2011) Efficiency of alchemical free energy simulations. I. A practical comparison of the exponential formula, thermodynamic integration, and Bennett’s acceptance ratio method. J Comput Chem 32(7):1303–1319. https://doi.org/10.1002/jcc.21713
Bruckner S, Boresch S (2011) Efficiency of alchemical free energy simulations. II. Improvements for thermodynamic integration. J Comput Chem 32(7):1320–1333. https://doi.org/10.1002/jcc.21712
Parameswaran S, Mobley DL (2014) Box size effects are negligible for solvation free energies of neutral solutes. J Comput Aided Mol Des 28(8):825–829. https://doi.org/10.1007/s10822-014-9766-7
Naden LN, Pham TT, Shirts MR (2014) Linear basis function approach to efficient alchemical free energy calculations. 1. Removal of uncharged atomic sites. J Chem Theory Comput 10(3):1128–1149. https://doi.org/10.1021/ct4009188
Naden LN, Shirts MR (2015) Linear basis function approach to efficient alchemical free energy calculations. 2. Inserting and deleting particles with Coulombic interactions. J Chem Theory Comput 11(6):2536–2549. https://doi.org/10.1021/ct501047e
Mecklenfeld A, Raabe G (2017) Efficient solvation free energy simulations: impact of soft-core potential and a new adaptive λ-spacing method. Mol Phys 115(9–12):1322–1334. https://doi.org/10.1080/00268976.2017.1292008
Bhati AP, Wan S, Hu Y, Sherborne B, Coveney PV (2018) Uncertainty quantification in alchemical free energy methods. J Chem Theory Comput 14(6):2867–2880. https://doi.org/10.1021/acs.jctc.7b01143
Yildirim A, Wassenaar TA, van der Spoel D (2018) Statistical efficiency of methods for computing free energy of hydration. J Chem Phys 149(14):144111. https://doi.org/10.1063/1.5041835
Wang X, Tu X, Zhang JZH, Sun Z (2018) BAR-based optimum adaptive sampling regime for variance minimization in alchemical transformation: the nonequilibrium stratification. Phys Chem Chem Phys 20(3):2009–2021. https://doi.org/10.1039/c7cp07573a
Shirts MR, Pande VS (2005) Comparison of efficiency and bias of free energies computed by exponential averaging, the Bennett acceptance ratio, and thermodynamic integration. J Chem Phys 122(14):144107. https://doi.org/10.1063/1.1873592
Shirts MR (2012) Best practices in free energy calculations for drug design. Methods Mol Biol 819:425–467. https://doi.org/10.1007/978-1-61779-465-0_26
Pohorille A, Jarzynski C, Chipot C (2010) Good practices in free-energy calculations. J Phys Chem B 114(32):10235–10253. https://doi.org/10.1021/jp102971x
Jambeck JPM, Mocci F, Lyubartsev AP, Laaksonen A (2013) Partial atomic charges and their impact on the free energy of solvation. J Comput Chem 34(3):187–197. https://doi.org/10.1002/jcc.23117
Mecklenfeld A, Raabe G (2017) Comparison of RESP and IPolQ-Mod partial charges for solvation free energy calculations of various solute/solvent pairs. J Chem Theory Comput 13(12):6266–6274. https://doi.org/10.1021/acs.jctc.7b00692
Nicholls A, Mobley DL, Guthrie JP, Chodera JD, Bayly CI, Cooper MD, Pande VS (2008) Predicting small-molecule solvation free energies: an informal blind test for computational chemistry. J Med Chem 51(4):769–779. https://doi.org/10.1021/jm070549+
Guthrie JP (2009) A blind challenge for computational solvation free energies: introduction and overview. J Phys Chem B 113(14):4501–4507. https://doi.org/10.1021/jp806724u
Geballe MT, Skillman AG, Nicholls A, Guthrie JP, Taylor PJ The SAMPL2 blind prediction challenge: introduction and overview. J Comput Aided Mol Des 24(4):259–279. https://doi.org/10.1007/s10822-010-9350-8
Mobley DL, Wymer KL, Lim NM, Guthrie JP (2014) Blind prediction of solvation free energies from the SAMPL4 challenge. J Comput Aided Mol Des 28(3):135–150. https://doi.org/10.1007/s10822-014-9718-2
Muddana HS, Fenley AT, Mobley DL, Gilson MK (2014) The SAMPL4 host–guest blind prediction challenge: an overview. J Comput Aided Mol Des 28(4):305–317. https://doi.org/10.1007/s10822-014-9735-1
Rizzo RC, Aynechi T, Case DA, Kuntz ID (2006) Estimation of absolute free energies of hydration using continuum methods: accuracy of partial charge models and optimization of nonpolar contributions. J Chem Theory Comput 2(1):128–139. https://doi.org/10.1021/ct050097l
Fennell CJ, Wymer KL, Mobley DL (2014) A fixed-charge model for alcohol polarization in the condensed phase, and its role in small molecule hydration. J Phys Chem B 118(24):6438–6446. https://doi.org/10.1021/jp411529h
Ahmed A, Sandler SI (2013) Hydration free energies of multifunctional nitroaromatic compounds. J Chem Theory Comput 9(6):2774–2785. https://doi.org/10.1021/ct3011002
Jämbeck JPM, Lyubartsev AP (2014) Update to the general amber force field for small solutes with an emphasis on free energies of hydration. J Phys Chem B 118(14):3793–3804. https://doi.org/10.1021/jp4111234
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174. https://doi.org/10.1002/jcc.20035
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J Am Chem Soc 118(45):11225–11236. https://doi.org/10.1021/ja9621760
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J, Darian E, Guvench O, Lopes P, Vorobyov I, MacKerell ADJR (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31(4):671–690. https://doi.org/10.1002/jcc.21367
Leontyev IV, Stuchebrukhov AA (2010) Electronic polarizability and the effective pair potentials of water. J Chem Theory Comput 6(10):3153–3161. https://doi.org/10.1021/ct1002048
Swope WC, Horn HW, Rice JE (2010) Accounting for polarization cost when using fixed charge force fields. I. Method for computing energy. J Phys Chem B 114(26):8621–8630. https://doi.org/10.1021/jp911699p
Lundborg M, Lindahl E (2015) Automatic GROMACS topology generation and comparisons of force fields for solvation free energy calculations. J Phys Chem B 119(3):810–823. https://doi.org/10.1021/jp505332p
Shi Y, Xia Z, Zhang J, Best R, Wu C, Ponder JW, Ren P (2013) The polarizable atomic multipole-based AMOEBA force field for proteins. J Chem Theory Comput 9(9):4046–4063. https://doi.org/10.1021/ct4003702
Lopes PEM, Huang J, Shim J, Luo Y, Li H, Roux B, MacKerell ADJR (2013) Force field for peptides and proteins based on the classical Drude oscillator. J Chem Theory Comput 9(12):5430–5449. https://doi.org/10.1021/ct400781b
Mohamed NA, Bradshaw RT, Essex JW (2016) Evaluation of solvation free energies for small molecules with the AMOEBA polarizable force field. J Comput Chem 37(32):2749–2758. https://doi.org/10.1002/jcc.24500
Cerutti DS, Rice JE, Swope WC, Case DA (2013) Derivation of fixed partial charges for amino acids accommodating a specific water model and implicit polarization. J Phys Chem B 117(8):2328–2338. https://doi.org/10.1021/jp311851r
Muddana HS, Sapra NV, Fenley AT, Gilson MK (2014) The SAMPL4 hydration challenge: evaluation of partial charge sets with explicit-water molecular dynamics simulations. J Comput Aided Mol Des 28(3):277–287. https://doi.org/10.1007/s10822-014-9714-6
Bayly CI, Cieplak P, Cornell W, Kollman PA (1993) A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J Phys Chem 97(40):10269–10280. https://doi.org/10.1021/j100142a004
Baker CM, Lopes PEM, Zhu X, Roux B, Mackerell AD (2010) Accurate calculation of hydration free energies using pair-specific Lennard-Jones parameters in the CHARMM Drude polarizable force field. J Chem Theory Comput 6(4):1181–1198. https://doi.org/10.1021/ct9005773
Loeffler HH, Bosisio S, Duarte Ramos Matos G, Suh D, Roux B, Mobley DL, Michel J (2018) Reproducibility of free energy calculations across different molecular simulation software packages. J Chem Theory Comput 14(11):5567–5582. https://doi.org/10.1021/acs.jctc.8b00544
Stroet M, Koziara KB, Malde AK, Mark AE (2017) Optimization of empirical force fields by parameter space mapping: a single-step perturbation approach. J Chem Theory Comput 13(12):6201–6212. https://doi.org/10.1021/acs.jctc.7b00800
Zwanzig RW (1954) High-temperature equation of state by a perturbation method. I. Nonpolar gases. J Chem Phys 22(8):1420. https://doi.org/10.1063/1.1740409
Koziara KB, Stroet M, Malde AK, Mark AE (2014) Testing and validation of the Automated Topology Builder (ATB) version 2.0: prediction of hydration free enthalpies. J Comput Aided Mol Des 28(3):221–233. https://doi.org/10.1007/s10822-014-9713-7
Bennett CH (1976) Efficient estimation of free energy differences from Monte Carlo data. J Comput Aided Mol Des 22(2):245–268. https://doi.org/10.1016/0021-9991(76)90078-4
Case DA, Berryman JT, Betz RM, Cerutti DS, Cheatham TE III, Darden TA, Duke RE, Giese TJ, Gohlke H, Gotz AW, Homeyer N, Izadi S, Janowski PA, Kaus JW, Kovalenko A, Lee T, Le Grand S, Li P, Luchko T, Luo R, Madej BD, Merz KM, Monard G, Needham H, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg AE, Salomon-Ferrer R, Simmerling C, Smith W, Swails J, Walker RC, Wang J, Wolf RM, Wu X, York DM, Kollman PA (2015) AMBER. University of California, San Francisco
Sousa da Silva AW, Vranken WF (2012) ACPYPE—AnteChamber PYthon Parser interfacE. BMC Res Notes 5:367. https://doi.org/10.1186/1756-0500-5-367
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02. Gaussian, Inc., Wallingford
Mennucci B, Cammi R, Tomasi J (1998) Excited states and solvatochromic shifts within a nonequilibrium solvation approach: a new formulation of the integral equation formalism method at the self-consistent field, configuration interaction, and multiconfiguration self-consistent field level. J Chem Phys 109(7):2798–2807. https://doi.org/10.1063/1.476878
Bekker H, Berendsen HJC, Dijkstra EJ, Achterop S, van Drunen R, van der Spoel D, Sijbers A, Keegstra H, Reitsma B, Renardus MKR (1993) GROMACS: a parallel computer for molecular dynamics simulations. In: de Groot RA, Nadrchal J (eds) Physics computing ’92
Berendsen HJC, van der Spoel D, van Drunen R (1995) GROMACS: a message-passing parallel molecular dynamics implementation. Comput Phys 91(1–3):43–56. https://doi.org/10.1016/0010-4655(95)00042-e
Lindahl E, Hess B, van der Spoel D (2001) GROMACS 3.0: a package for molecular simulation and trajectory analysis. J Mol Model 7(8):306–317. https://doi.org/10.1007/s008940100045
van der Spoel D, Lindahl E, Hess B, Groenhof G, Mark AE, Berendsen HJ (2005) GROMACS: fast, flexible, and free. J Comput Chem 26(16):1701–1718. https://doi.org/10.1002/jcc.20291
Hess B, Kutzner C, van der Spoel D, Lindahl E (2008) GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J Chem Theory Comput 4(3):435–447. https://doi.org/10.1021/ct700301q
Pronk S, Páll S, Schulz R, Larsson P, Bjelkmar P, Apostolov R, Shirts MR, Smith JC, Kasson PM, van der Spoel D, Hess B, Lindahl E (2013) GROMACS 4.5: a high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 29(7):845–854. https://doi.org/10.1093/bioinformatics/btt055
Páll S, Abraham MJ, Kutzner C, Hess B, Lindahl E (2015) Tackling Exascale software challenges in molecular dynamics simulations with GROMACS. In: Markidis S, Laure E (eds) Solving software challenges for Exascale, vol 8759. Springer, Cham, pp 3–27
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2:19–25. https://doi.org/10.1016/j.softx.2015.06.001
Goga N, Rzepiela AJ, de Vries AH, Marrink SJ, Berendsen HJC (2012) Efficient algorithms for Langevin and DPD dynamics. J Chem Theory Comput 8(10):3637–3649. https://doi.org/10.1021/ct3000876
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684. https://doi.org/10.1063/1.448118
Parrinello M, Rahman A (1980) Crystal structure and pair potentials: a molecular-dynamics study. Phys Rev Lett 45(14):1196–1199. https://doi.org/10.1103/physrevlett.45.1196
Martinez L, Andrade R, Birgin EG, Martinez JM (2009) PACKMOL: a package for building initial configurations for molecular dynamics simulations. J Comput Chem 30(13):2157–2164. https://doi.org/10.1002/jcc.21224
Beutler TC, Mark AE, van Schaik RC, Gerber PR, van Gunsteren WF (1994) Avoiding singularities and numerical instabilities in free energy calculations based on molecular simulations. Chem Phys Lett 222(6):529–539. https://doi.org/10.1016/0009-2614(94)00397-1
Pham TT, Shirts MR (2011) Identifying low variance pathways for free energy calculations of molecular transformations in solution phase. J Chem Phys 135(3):34114. https://doi.org/10.1063/1.3607597
Shirts MR, Chodera JD (2008) Statistically optimal analysis of samples from multiple equilibrium states. J Chem Phys 129(12):124105. https://doi.org/10.1063/1.2978177
Efron B (1979) Bootstrap methods: another look at the jackknife. Ann Statist 7(1):1–26. https://doi.org/10.1214/aos/1176344552
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68(3):441–451. https://doi.org/10.1021/j100785a001
Eisenhaber F, Lijnzaad P, Argos P, Sander C, Scharf M (1995) The double cubic lattice method: efficient approaches to numerical integration of surface area and volume and to dot surface contouring of molecular assemblies. J Comput Chem 16(3):273–284. https://doi.org/10.1002/jcc.540160303
Acknowledgements
Andreas Mecklenfeld acknowledges funding by a Georg-Christoph-Lichtenberg Fellowship by the Federal State of Lower Saxony, Germany. The authors declare no competing financial interest. Molecular dynamics simulations were performed with resources provided by the North-German Supercomputing Alliance (HLRN). We greatly appreciate the support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mecklenfeld, A., Raabe, G. Applicability of a thermodynamic cycle approach for a force field parametrization targeting non-aqueous solvation free energies. J Comput Aided Mol Des 34, 71–82 (2020). https://doi.org/10.1007/s10822-019-00261-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-019-00261-5