Abstract
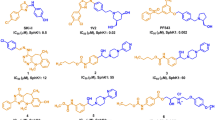
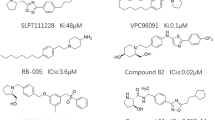
Activity cliffs (ACs) are defined as closely analogous compounds of significant affinity discrepancies against certain biotarget. In this paper we propose to use AC pair(s) for extracting valid binding pharmacophores through exposing corresponding protein complexes to stochastic deformation/relaxation followed by applying genetic algorithm/machine learning (GA-ML) for selecting optimal pharmacophore(s) that best classify a long list of inhibitors. We compared the performances of ligand-based and structure-based pharmacophores with counterparts generated by this newly introduced technique. Sphingosine kinase 1 (SPHK-1) was used as case study. SPHK-1 is a lipid kinase that plays pivotal role in the regulation of a variety of biological processes including, cell growth, apoptosis, and inflammation. The new approach proved to yield pharmacophore and ML models of comparable accuracies to established ligand-based and structure-based pharmacophores. The resulting pharmacophores and ML models were used to capture hits from the national cancer institute list of compounds and predict their bioactivity categories. Two hits of novel chemotypes showed selective and low micromolar inhibitory IC50 values against SPHK-1.










Similar content being viewed by others
References
Stumpfe D, Hu H, Bajorath J (2020) Advances in exploring activity cliffs. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-020-00315-z
Heikamp K, Hu X, Yan A, Bajorath J (2012) Prediction of activity cliffs using support vector machines. J Chem Inf Model. https://doi.org/10.1021/ci300306a
Namasivayam V, Bajorath J (2012) Searching for coordinated activity cliffs using particle swarm optimization. J Chem Inf Model. https://doi.org/10.1021/ci3000503
Namasivayam V, Iyer P, Bajorath J (2013) Prediction of individual compounds forming activity cliffs using emerging chemical patterns. J Chem Inf Model. https://doi.org/10.1021/ci400597d
Guha R (2012) Exploring uncharted territories: predicting activity cliffs in structure-activity landscapes. J Chem Inf Model. https://doi.org/10.1021/ci300047k
Seebeck B, Wagener M, Rarey M (2011) From activity cliffs to target-specific scoring models and pharmacophore hypotheses. ChemMedChem. https://doi.org/10.1002/cmdc.201100179
Hu Y, Bajorath J, Stumpfe D (2013) Advancing the activity cliff concept. F1000Research. https://doi.org/10.12688/f1000research.2-199.v1
Rühmann E, Betz M, Heine A, Klebe G (2015) Fragment binding can be either more enthalpy-driven or entropy-driven: crystal structures and residual hydration patterns suggest why. J Med Chem. https://doi.org/10.1021/acs.jmedchem.5b00812
Biela A, Betz M, Heine A, Klebe G (2012) Water makes the difference: rearrangement of water solvation layer triggers non-additivity of functional group contributions in protein-ligand binding. ChemMedChem. https://doi.org/10.1002/cmdc.201200206
Krimmer SG, Betz M, Heine A, Klebe G (2014) Methyl, ethyl, propyl, butyl: futile but not for water, as the correlation of structure and thermodynamic signature shows in a congeneric series of thermolysin inhibitors. ChemMedChem. https://doi.org/10.1002/cmdc.201400013
Reddy M, Reddy C, Rathore R, Erion M, Aparoy P, Reddy R et al (2014) Free energy calculations to estimate ligand-binding affinities in structure-based drug design. Curr Pharm Des. https://doi.org/10.2174/13816128113199990604
Gkeka P, Eleftheratos S, Kolocouris A, Cournia Z (2013) Free energy calculations reveal the origin of binding preference for aminoadamantane blockers of influenza A/M2TM pore. J Chem Theory Comput. https://doi.org/10.1021/ct300899n
Christ CD, Fox T (2014) Accuracy assessment and automation of free energy calculations for drug design. J Chem Inf Model. https://doi.org/10.1021/ci4004199
Wang L, Wu Y, Deng Y, Kim B, Pierce L, Krilov G et al (2015) Accurate and reliable prediction of relative ligand binding potency in prospective drug discovery by way of a modern free-energy calculation protocol and force field. J Am Chem Soc. https://doi.org/10.1021/ja512751q
Medina-Franco JL, Méndez-Lucio O, Martinez-Mayorga K (2014). The interplay between molecular modeling and chemoinformatics to characterize protein-ligand and protein-protein interactions landscapes for drug discovery. In: Advances in protein chemistry and structural biology. https://doi.org/10.1016/bs.apcsb.2014.06.001
Pérez-Benito L, Casajuana-Martin N, Jiménez-Rosés M, Van Vlijmen H, Tresadern G (2019) Predicting activity cliffs with free-energy perturbation. J Chem Theory Comput. https://doi.org/10.1021/acs.jctc.8b01290
Daoud S, Taha MO (2020) Pharmacophore modeling of JAK1: a target infested with activity-cliffs. J Mol Graph Model. https://doi.org/10.1016/j.jmgm.2020.107615
Maggiora GM (2006) On outliers and activity cliffs—why QSAR often disappoints. J Chem Inf Model. https://doi.org/10.1021/ci060117s
Stumpfe D, Bajorath J (2012) Exploring activity cliffs in medicinal chemistry. J Med Chem. https://doi.org/10.1021/jm201706b
Bajorath J (2012) Modeling of activity landscapes for drug discovery. Exp Opin Drug Disc. https://doi.org/10.1517/17460441.2012.679616
Peltason L, Bajorath J (2007) SAR index: quantifying the nature of structure-activity relationships. J Med Chem. https://doi.org/10.1021/jm0705713
Guha R, Van Drie JH (2008) Structure—activity landscape index: Identifying and quantifying activity cliffs. J Chem Inf Model. https://doi.org/10.1021/ci7004093
Vogt M, Huang Y, Bajorath J (2011) From activity cliffs to activity ridges: Informative data structures for SAR analysis. J Chem Inf Model. https://doi.org/10.1021/ci2002473
Hu Y, Bajorath J (2012) Extending the activity cliff concept: Structural categorization of activity cliffs and systematic identification of different types of cliffs in the ChEMBL database. J Chem Inf Model. https://doi.org/10.1021/ci300274c
Klebe G (2019) Broad-scale analysis of thermodynamic signatures in medicinal chemistry: are enthalpy-favored binders the better development option? Drug Discov Today. https://doi.org/10.1016/j.drudis.2019.01.014
Amaral M, Kokh DB, Bomke J, Wegener A, Buchstaller HP, Eggenweiler HM et al (2017) Protein conformational flexibility modulates kinetics and thermodynamics of drug binding. Nat Commun. https://doi.org/10.1038/s41467-017-02258-w
Koch C, Heine A, Klebe G (2011) Ligand-induced fit affects binding modes and provokes changes in crystal packing of aldose reductase. Biochim Biophys Acta Gen Subj. https://doi.org/10.1016/j.bbagen.2011.06.001
Steuber H, Czodrowski P, Sotriffer CA, Klebe G (2007) Tracing changes in protonation: a prerequisite to factorize thermodynamic data of inhibitor binding to aldose reductase. J Mol Biol. https://doi.org/10.1016/j.jmb.2007.08.063
Steuber H, Heine A, Klebe G (2007) Structural and thermodynamic study on aldose reductase: nitro-substituted inhibitors with strong enthalpic binding contribution. J Mol Biol. https://doi.org/10.1016/j.jmb.2006.12.004
Ehrmann FR, Stojko J, Metz A, Debaene F, Barandun LJ, Heine A et al (2017) Soaking suggests “alternative facts”: Only cocrystallization discloses major ligand-induced interface rearrangements of a homodimeric tRNA-binding protein indicating a novel mode-of-inhibition. PLoS ONE. https://doi.org/10.1371/journal.pone.0175723
Zubrienė A, Smirnov A, Dudutienė V, Timm DD, Matulienė J, Michailovienė V et al (2016) Intrinsic thermodynamics and structures of 2,4- and 3,4-substituted fluorinated benzenesulfonamides binding to carbonic anhydrases. ChemMedChem. https://doi.org/10.1002/cmdc.201600509
Kisonaite M, Zubriene A, Capkauskaite E, Smirnov A, Smirnoviene J, Kairys V et al (2014) Intrinsic thermodynamics and structure correlation of benzenesulfonamides with a pyrimidine moiety binding to carbonic anhydrases I, II, VII, XII, and XIII. PLoS ONE. https://doi.org/10.1371/journal.pone.0114106
DuBay KH, Geissler PL (2009) Calculation of proteins’ total side-chain torsional entropy and its influence on protein-ligand interactions. J Mol Biol. https://doi.org/10.1016/j.jmb.2009.05.068
Zhang J, Liu JS (2006) On side-chain conformational entropy of proteins. PLoS Comput Biol. https://doi.org/10.1371/journal.pcbi.0020168
Doig AJ, Sternberg MJE (1995) Side-chain conformational entropy in protein folding. Protein Sci. https://doi.org/10.1002/pro.5560041101
Plattner N, Noé F (2015) Protein conformational plasticity and complex ligand-binding kinetics explored by atomistic simulations and Markov models. Nat Commun. https://doi.org/10.1038/ncomms8653
Hatmal MM, Taha MO (2018) Combining stochastic deformation/relaxation and intermolecular contacts analysis for extracting pharmacophores from ligand-receptor complexes. J Chem Inf Model. https://doi.org/10.1021/acs.jcim.7b00708
Leach AR, Gillet VJ, Lewis RA, Taylor R (2010) Three-dimensional pharmacophore methods in drug discovery. J Med Chem. https://doi.org/10.1021/jm900817u
Tuffaha GO, Hatmal MM, Taha MO (2019) Discovery of new JNK3 inhibitory chemotypes via QSAR-Guided selection of docking-based pharmacophores and comparison with other structure-based pharmacophore modeling methods. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2019.05.015
Sliwoski G, Kothiwale S, Meiler J, Lowe EW (2014) Computational methods in drug discovery. Pharmacol Rev. https://doi.org/10.1124/pr.112.007336
Al-Sha’er MA, Taha MO (2018) Ligand-based modeling of Akt3 lead to potent dual Akt1/Akt3 inhibitor. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2018.02.001
Paquet E, Viktor HL (2015) Molecular dynamics, Monte Carlo simulations, and langevin dynamics: a computational review. Biomed Res Int. https://doi.org/10.1155/2015/183918
Ogretmen B (2017) Sphingolipid metabolism in cancer signaling and therapy. Nat Rev Cancer. https://doi.org/10.1038/nrc.2017.96
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2011.09.003
Pulkoski-Gross MJ, Obeid LM (2018) Molecular mechanisms of regulation of sphingosine kinase 1. Biochimica et Biophysica Acta Mol Cell Biol Lipids. https://doi.org/10.1016/j.bbalip.2018.08.015
Bocheńska K, Gabig-Cimińska M (2020) Unbalanced sphingolipid metabolism and its implications for the pathogenesis of psoriasis. Molecules. https://doi.org/10.3390/molecules25051130
Gomez-Larrauri A, Presa N, Dominguez-Herrera A, Ouro A, Trueba M, Gomez-Munoz A (2020) Role of bioactive sphingolipids in physiology and pathology. Essays Biochem. https://doi.org/10.1042/EBC20190091
Kroll A, Cho HE, Kang MH (2020) Antineoplastic agents targeting sphingolipid pathways. Front Oncol. https://doi.org/10.3389/fonc.2020.00833
Spiegel S (2020) Sphingosine-1-phosphate: from insipid lipid to a key regulator. J Biol Chem. https://doi.org/10.1074/jbc.X120.012838
Al-Sha’er MA, Taha MO (2020) Elaboration of novel TTK1 inhibitory leads via QSAR-guided selection of crystallographic pharmacophores followed by in vitro assay. Curr Comput Aided Drug Des. https://doi.org/10.2174/1573409916666200611122736
Al-Sha’er MA, Mansi I, Khanfar M, Abudayyh A (2016) Discovery of new heat shock protein 90 inhibitors using virtual co-crystallized pharmacophore generation. J Enzyme Inhib Med Chem. https://doi.org/10.1080/14756366.2016.1218485
Taha MO, Habash M, Hatmal MM, Abdelazeem AH, Qandil A (2015) Ligand-based modeling followed by in vitro bioassay yielded new potent glucokinase activators. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2014.12.003
Alabed SJ, Khanfar M, Taha MO (2016) Computer-aided discovery of new FGFR-1 inhibitors followed by in vitro validation. Future Med Chem. https://doi.org/10.4155/fmc-2016-0056
Al-Aqtash RA, Zihlif MA, Hammad H, Nassar ZD, Al MJ, Taha MO (2017) Ligand-based computational modelling of platelet-derived growth factor beta receptor leading to new angiogenesis inhibitory leads. Comput Biol Chem. https://doi.org/10.1016/j.compbiolchem.2017.10.003
Kurogi Y, Guner O (2012) Pharmacophore modeling and three-dimensional database searching for drug design using catalyst. Curr Med Chem. https://doi.org/10.2174/0929867013372481
Taha MO, Dahabiyeh LA, Bustanji Y, Zalloum H, Saleh S (2008) Combining ligand-based pharmacophore modeling, quantitative structure-activity relationship analysis and in silico screening for the discovery of new potent hormone sensitive lipase inhibitors. J Med Chem. https://doi.org/10.1021/jm800718k
Abuhammad AM, Taha MO (2009) Pharmacophore modeling, quantitative structure—activity relationship analysis, and shape-complemented in silico screening allow access to novel influenza neuraminidase inhibitors. J Chem Inf Model. https://doi.org/10.1021/ci8003682
Taha MO, Al-Sha’Er MA, Khanfar MA, Al-Nadaf AH (2014) Discovery of nanomolar phosphoinositide 3-kinase gamma (PI3Kγ) inhibitors using ligand-based modeling and virtual screening followed by in vitro analysis. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2014.07.056
Habash M, Abdelazeem AH, Taha MO (2014) Elaborate ligand-based modeling reveals new human neutrophil elastase inhibitors. Med Chem Res. https://doi.org/10.1007/s00044-014-0966-4
Abu Khalaf R, Abu Sheikha G, Bustanji Y, Taha MO (2010) Discovery of new cholesteryl ester transfer protein inhibitors via ligand-based pharmacophore modeling and QSAR analysis followed by synthetic exploration. Eur J Med Chem. https://doi.org/10.1016/j.ejmech.2009.12.070
Abuhamdah S, Habash M, Taha MO (2013) Elaborate ligand-based modeling coupled with QSAR analysis and in silico screening reveal new potent acetylcholinesterase inhibitors. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-013-9699-6
Kirchmair J, Laggner C, Wolber G, Langer T (2005) Comparative analysis of protein-bound ligand conformations with respect to catalyst’s conformational space subsampling algorithms. J Chem Inf Model. https://doi.org/10.1021/ci0497531
Li J, Ehlers T, Sutter J, Varma-O’Brien S, Kirchmair J (2007) CAESAR: a new conformer generation algorithm based on recursive buildup and local rotational symmetry consideration. J Chem Inf Model. https://doi.org/10.1021/ci700136x
Vanommeslaeghe K, Hatcher E, Acharya C, Kundu S, Zhong S, Shim J et al (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem. https://doi.org/10.1002/jcc.21367
Abutayeh RF, Taha MO (2019) Discovery of novel Flt3 inhibitory chemotypes through extensive ligand-based and new structure-based pharmacophore modelling methods. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2019.01.011
Poptodorov K, Luu T, Hoffmann RD (2006) Pharmacophore model generation software tools. In: Pharmacophores and pharmacophore searches. https://doi.org/10.1002/3527609164.ch2
Guner O, Clement O, Kurogi Y (2004) Pharmacophore modeling and three dimensional database searching for drug design using catalyst: recent advances. Curr Med Chem. https://doi.org/10.2174/0929867043364036
Shahin R, AlQtaishat S, Taha MO (2012) Elaborate ligand-based modeling reveal new submicromolar Rho kinase inhibitors. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-011-9509-y
Pandey A, Paliwal SK, Paliwal SK (2014) Chemical feature-based molecular modeling of urotensin-II receptor antagonists: generation of predictive pharmacophore model for early drug discovery. J Chem. https://doi.org/10.1155/2014/921863
Basu D (1980) Randomization analysis of experimental data: the fisher randomization test. J Am Stat Assoc. https://doi.org/10.1080/01621459.1980.10477512
Taha MO (2012) Mixing pharmacophore modeling and classical QSAR analysis as powerful tool for lead discovery. In: Virtual screening. https://doi.org/10.5772/20993
Khanfar MA, AbuKhader MM, Alqtaishat S, Taha MO (2013) Pharmacophore modeling, homology modeling, and in silico screening reveal mammalian target of rapamycin inhibitory activities for sotalol, glyburide, metipranolol, sulfamethizole, glipizide, and pioglitazone. J Chem Inf Model. https://doi.org/10.1016/j.jmgm.2013.02.009
Khanfar MA, Taha MO (2017) Unsupervised pharmacophore modeling combined with QSAR analyses revealed novel low micromolar SIRT2 inhibitors. J Mol Recognit. https://doi.org/10.1002/jmr.2623
Taha MO, Bustanji Y, Al-Bakri AG, Yousef AM, Zalloum WA, Al-Masri IM et al (2007) Discovery of new potent human protein tyrosine phosphatase inhibitors via pharmacophore and QSAR analysis followed by in silico screening. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2006.08.008
Kennedy AJ, Mathews TP, Kharel Y, Field SD, Moyer ML, East JE et al (2011) Development of amidine-based sphingosine kinase 1 nanomolar inhibitors and reduction of sphingosine 1-phosphate in human leukemia cells. J Med Chem. https://doi.org/10.1021/jm2001053
Congdon MD, Kharel Y, Brown AM, Lewis SN, Bevan DR, Lynch KR et al (2016) Structure-activity relationship studies and molecular modeling of naphthalene-based sphingosine kinase 2 inhibitors. ACS Med Chem Lett. https://doi.org/10.1021/acsmedchemlett.5b00304
Houck JD, Dawson TK, Kennedy AJ, Kharel Y, Naimon ND, Field SD et al (2016) Structural requirements and docking analysis of amidine-based sphingosine kinase 1 inhibitors containing oxadiazoles. ACS Med Chem Lett. https://doi.org/10.1021/acsmedchemlett.6b00002
Aurelio L, Scullino CV, Pitman MR, Sexton A, Oliver V, Davies L et al (2016) From sphingosine kinase to dihydroceramide desaturase: a structure-activity relationship (SAR) study of the enzyme inhibitory and anticancer activity of 4-((4-(4-chlorophenyl)thiazol-2-yl)amino)phenol (SKI-II). J Med Chem. https://doi.org/10.1021/acs.jmedchem.5b01439
Xi M, Ge J, Wang X, Sun C, Liu T, Fang L et al (2016) Development of hydroxy-based sphingosine kinase inhibitors and anti-inflammation in dextran sodium sulfate induced colitis in mice. Bioorgan Med Chem. https://doi.org/10.1016/j.bmc.2016.05.047
Plano D, Amin S, Sharma AK (2014) Importance of sphingosine kinase (SphK) as a target in developing cancer therapeutics and recent developments in the synthesis of novel SphK inhibitors. J Med Chem. https://doi.org/10.1021/jm4011687
Patwardhan NN, Morris EA, Kharel Y, Raje MR, Gao M, Tomsig JL et al (2015) Structure-activity relationship studies and in vivo activity of guanidine-based sphingosine kinase inhibitors: discovery of SphK1- and SphK2-selective inhibitors. J Med Chem. https://doi.org/10.1021/jm501760d
Hengst JA, Wang X, Sk UH, Sharma AK, Amin S, Yun JK (2010) Development of a sphingosine kinase 1 specific small-molecule inhibitor. Bioorgan Med Chem Lett. https://doi.org/10.1016/j.bmcl.2010.10.005
Childress ES, Kharel Y, Brown AM, Bevan DR, Lynch KR, Santos WL (2017) Transforming sphingosine kinase 1 inhibitors into dual and sphingosine kinase 2 selective inhibitors: design, synthesis, and in vivo activity. J Med Chem. https://doi.org/10.1021/acs.jmedchem.7b00233
Al-Barghouthy E, Abuhammad A, Taha MO (2019) QSAR-guided pharmacophore modeling and subsequent virtual screening identify novel TYK2 inhibitor. Med Chem Res. https://doi.org/10.1007/s00044-019-02377-7
Xiang Y, Hirth B, Kane JL, Liao J, Noson KD, Yee C et al (2010) Discovery of novel sphingosine kinase-1 inhibitors. Part 2. Bioorgan Med Chem Lett. https://doi.org/10.1016/j.bmcl.2010.06.019
Qu W, Ploessl K, Truong H, Kung MP, Kung HF (2009) Iodophenyl tagged sphingosine derivatives: synthesis and preliminary biological evaluation. Bioorgan Med Chem Lett. https://doi.org/10.1016/j.bmcl.2009.05.035
Byun HS, Pyne S, MacRitchie N, Pyne NJ, Bittman R (2013) Novel sphingosine-containing analogues selectively inhibit sphingosine kinase (SK) isozymes, induce SK1 proteasomal degradation and reduce DNA synthesis in human pulmonary arterial smooth muscle cells. Medchemcomm. https://doi.org/10.1039/c3md00201b
Baek DJ, MacRitchie N, Anthony NG, MacKay SP, Pyne S, Pyne NJ et al (2013) Structure-activity relationships and molecular modeling of sphingosine kinase inhibitors. J Med Chem. https://doi.org/10.1021/jm401399c
Ohno H, Honda M, Hamada N, Miyagaki J, Iwata A, Otsuki K et al (2017) Identification of selective inhibitors of sphingosine kinases 1 and 2 through a structure–activity relationship study of 4-epi-jaspine B. Bioorgan Med Chem. https://doi.org/10.1016/j.bmc.2017.03.059
Schnute ME, McReynolds MD, Carroll J, Chrencik J, Highkin MK, Iyanar K et al (2017) Discovery of a potent and selective sphingosine kinase 1 inhibitor through the molecular combination of chemotype-distinct screening hits. J Med Chem. https://doi.org/10.1021/acs.jmedchem.7b00070
Gustin DJ, Li Y, Brown ML, Min X, Schmitt MJ, Wanska M et al (2013) Structure guided design of a series of sphingosine kinase (SphK) inhibitors. Bioorgan Med Chem Lett. https://doi.org/10.1016/j.bmcl.2013.06.030
Xiang Y, Asmussen G, Booker M, Hirth B, Kane JL, Liao J et al (2009) Discovery of novel sphingosine kinase 1 inhibitors. Bioorgan Med Chem Lett. https://doi.org/10.1016/j.bmcl.2009.09.022
Al-Sha’er MA, Mansi I, Almazari I, Hakooz N (2015) Evaluation of novel Akt1 inhibitors as anticancer agents using virtual co-crystallized pharmacophore generation. J Mol Gr Model. https://doi.org/10.1016/j.jmgm.2015.10.004
Rao SN, Head MS, Kulkarni A, LaLonde JM (2007) Validation studies of the site-directed docking program LibDock. J Chem Inf Model. https://doi.org/10.1021/ci6004299
Diller DJ, Merz KM (2001) High throughput docking for library design and library prioritization. Proteins Struct Funct Genet 43:113–124
Alam S, Khan F (2018) Virtual screening, docking, ADMET and system pharmacology studies on garcinia caged xanthone derivatives for anticancer activity. Sci Rep. https://doi.org/10.1038/s41598-018-23768-7
Venkatachalam CM, Jiang X, Oldfield T, Waldman M (2003) LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol Gr Model. https://doi.org/10.1016/S1093-3263(02)00164-X
Wu G, Robertson DH, Brooks CL, Vieth M (2003) Detailed analysis of grid-based molecular docking: a case study of CDOCKER—a CHARMm-based MD docking algorithm. J Comput Chem. https://doi.org/10.1002/jcc.10306
Jain A (2006) Scoring functions for protein-ligand docking. Curr Protein Pept Sci. https://doi.org/10.2174/138920306778559395
Gehlhaar DK, Verkhivker GM, Rejto PA, Sherman CJ, Fogel DR, Fogel LJ et al (1995) Molecular recognition of the inhibitor AG-1343 by HIV-1 protease: conformationally flexible docking by evolutionary programming. Chem Biol. https://doi.org/10.1016/1074-5521(95)90050-0
Muegge I, Martin YC (1999) A general and fast scoring function for protein-ligand interactions: a simplified potential approach. J Med Chem. https://doi.org/10.1021/jm980536j
Muegge I (2002) Pharmacophore features of potential drugs. Chem Eur J 8:1976–1981
Hussain J, Rea C (2010) Computationally efficient algorithm to identify matched molecular pairs (MMPs) in large data sets. J Chem Inf Model. https://doi.org/10.1021/ci900450m
Carpenter KA, Huang X (2018) Machine learning-based virtual screening and its applications to Alzheimer’s drug discovery: a review. Curr Pharm Des. https://doi.org/10.2174/1381612824666180607124038
Lavecchia A (2015) Machine-learning approaches in drug discovery: methods and applications. Drug Discov. https://doi.org/10.1016/j.drudis.2014.10.012
Cano G, Garcia-Rodriguez J, Garcia-Garcia A, Perez-Sanchez H, Benediktsson JA, Thapa A et al (2017) Automatic selection of molecular descriptors using random forest: application to drug discovery. Expert Syst Appl. https://doi.org/10.1016/j.eswa.2016.12.008
Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G et al (2019) Applications of machine learning in drug discovery and development. Nat Rev Drug Discov. https://doi.org/10.1038/s41573-019-0024-5
Wickramasinghe I, Kalutarage H (2020) Naive Bayes: applications, variations and vulnerabilities: a review of literature with code snippets for implementation. Soft Comput. https://doi.org/10.1007/s00500-020-05297-6
Zhang H, Yu P, Ren JX, Li XB, Wang HL, Ding L et al (2017) Development of novel prediction model for drug-induced mitochondrial toxicity by using naïve Bayes classifier method. Food Chem Toxicol. https://doi.org/10.1016/j.fct.2017.10.021
Zhang H, Liu CT, Mao J, Shen C, Xie RL, Mu B (2020) Development of novel in silico prediction model for drug-induced ototoxicity by using naïve Bayes classifier approach. Toxicol Vitr. https://doi.org/10.1016/j.tiv.2020.104812
Derksen S, Rau O, Schneider P, Schubert-Zsilavecz M, Schneider G (2006) Virtual screening for PPAR modulators using a probabilistic neural network. ChemMedChem. https://doi.org/10.1002/cmdc.200600166
Wang SL, Li X, Zhang S, Gui J, Huang DS (2010) Tumor classification by combining PNN classifier ensemble with neighborhood rough set based gene reduction. Comput Biol Med. https://doi.org/10.1016/j.compbiomed.2009.11.014
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochem Med. https://doi.org/10.11613/bm.2012.031
Hatmal MM, Abuyaman O, Taha MO (2021) Docking-generated multiple ligand poses for bootstrapping bioactivity classifying machine learning: repurposing covalent inhibitors for COVID-19-related TMPRSS2 as case study. Comput Struct Biotechnol J. https://doi.org/10.1016/j.csbj.2021.08.023
Kirchmair J, Markt P, Distinto S, Wolber G, Langer T (2008) Evaluation of the performance of 3D virtual screening protocols: RMSD comparisons, enrichment assessments, and decoy selection—what can we learn from earlier mistakes? J Comput Aided Mol Des. https://doi.org/10.1007/s10822-007-9163-6
Taha MO, Habash M, Khanfar MA (2014) The use of docking-based comparative intermolecular contacts analysis to identify optimal docking conditions within glucokinase and to discover of new GK activators. J Comput-Aided Mol Des. https://doi.org/10.1007/s10822-014-9740-4
Triballeau N, Acher F, Brabet I, Pin JP, Bertrand HO (2005) Virtual screening workflow development guided by the “receiver operating characteristic” curve approach. Application to high-throughput docking on metabotropic glutamate receptor subtype 4. J Med Chem 1:1. https://doi.org/10.1021/jm049092j
Kondeti PK, Ravi K, Mutheneni SR, Kadiri MR, Kumaraswamy S, Vadlamani R et al (2019) Applications of machine learning techniques to predict filariasis using socio-economic factors. Epidemiol Infect. https://doi.org/10.1017/S0950268819001481
Vehtari A, Gelman A, Gabry J (2017) Practical Bayesian model evaluation using leave-one-out cross-validation and WAIC. Stat Comput. https://doi.org/10.1007/s11222-016-9696-4
Rogers D, Hopfinger AJ (1994) Application of genetic function approximation to quantitative structure-activity relationships and quantitative structure-property relationships. J Chem Inf Comput Sci. https://doi.org/10.1021/ci00020a020
Chan JCW, Paelinckx D (2008) Evaluation of random forest and adaboost tree-based ensemble classification and spectral band selection for ecotope mapping using airborne hyperspectral imagery. Remote Sens Environ. https://doi.org/10.1016/j.rse.2008.02.011
James G, Witten D, Hastie T, Tibishirani R (2013) An introduction to statistical learning with applications in R (older version). Springer Texts in Statistics.
Hatmal MM, Taha MO (2017) Simulated annealing molecular dynamics and ligand-receptor contacts analysis for pharmacophore modeling. Future Med Chem. https://doi.org/10.4155/fmc-2017-0061
Hatmal MM, Jaber S, Taha MO (2016) Combining molecular dynamics simulation and ligand-receptor contacts analysis as a new approach for pharmacophore modeling: beta-secretase 1 and check point kinase 1 as case studies. J Comput Aided Mol Des. https://doi.org/10.1007/s10822-016-9984-2
Hijjawi MS, Abutayeh RF, Taha MO (2020) Structure-based discovery and bioactivity evaluation of novel aurora-A kinase inhibitors as anticancer agents via docking-based comparative intermolecular contacts analysis (dbCICA). Molecules. https://doi.org/10.3390/molecules25246003
Al-Nadaf AH, Salah SA, Taha MO (2018) Discovery of new Gyrase β inhibitors via structure based modeling. Comput Biol Chem. https://doi.org/10.1016/j.compbiolchem.2018.03.020
Habash M, Abuhamdah S, Younis K, Taha MO (2017) Docking-based comparative intermolecular contacts analysis and in silico screening reveal new potent acetylcholinesterase inhibitors. Med Chem Res. https://doi.org/10.1007/s00044-017-1976-9
Shahin R, Taha MO (2012) Elaborate ligand-based modeling and subsequent synthetic exploration unveil new nanomolar Ca2+/calmodulin-dependent protein kinase II inhibitory leads. Bioorgan Med Chem. https://doi.org/10.1016/j.bmc.2011.10.071
Kashem MA, Nelson RM, Yingling JD, Pullen SS, Prokopowicz AS, Jones JW et al (2007) Three mechanistically distinct kinase assays compared: Measurement of intrinsic ATPase activity identified the most comprehensive set of ITK inhibitors. J Biomol Screen. https://doi.org/10.1177/1087057106296047
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G et al (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Meloun M, Militký J, Hill M, Brereton RG (2002) Crucial problems in regression modelling and their solutions. Analyst. https://doi.org/10.1039/b110779h
Schnute ME, McReynolds MD, Kasten T, Yates M, Jerome G, Rains JW et al (2012) Modulation of cellular S1P levels with a novel, potent and specific inhibitor of sphingosine kinase-1. Biochem J. https://doi.org/10.1042/BJ20111929
Shoichet B (2006) Interpreting steep dose-response curves in early inhibitor discovery. J Med Chem. https://doi.org/10.1021/jm061103g
Siddamurthi S, Gutti G, Jana S, Kumar A, Singh SK (2020) Anthraquinone: a promising scaffold for the discovery and development of therapeutic agents in cancer therapy. Future Med Chem. https://doi.org/10.4155/fmc-2019-0198
Stasevych M, Zvarych V, Lunin V, Halenova T, Savchuk O, Dudchak O et al (2015) Novel anthraquinone-based derivatives as potent inhibitors for receptor tyrosine kinases. Indian J Pharm Sci. https://doi.org/10.4103/0250-474X.169062
Liang Z, Ai J, Ding X, Peng X, Zhang D, Zhang R et al (2013) Anthraquinone derivatives as potent inhibitors of c-Met kinase and the extracellular signaling pathway. ACS Med Chem Lett. https://doi.org/10.1021/ml4000047
Balaban AT (1982) Highly discriminating distance-based topological index. Chem Phys Lett. https://doi.org/10.1016/0009-2614(82)80009-2
Keir M, Hall L (1976) Molecular connectivity in chemistry and drug research. Academic Press, New York
Acknowledgements
The authors thank the Deanship of Scientific Research at the University of Jordan for funding this project. The authors are also indebted for the Science Research Fund / Jordan Ministry of Higher Education for their generous grant.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mousa, L.A., Hatmal, M.M. & Taha, M. Exploiting activity cliffs for building pharmacophore models and comparison with other pharmacophore generation methods: sphingosine kinase 1 as case study. J Comput Aided Mol Des 36, 39–62 (2022). https://doi.org/10.1007/s10822-021-00435-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10822-021-00435-0