Abstract
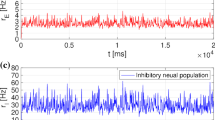
Individual neurons in the suprachiasmatic nucleus (SCN), the master biological clock in mammals, autonomously produce highly complex patterns of spikes. We have shown that most (~90%) SCN neurons exhibit truly stochastic interspike interval (ISI) patterns. The aim of this study was to understand the stochastic nature of the firing patterns in SCN neurons by analyzing the ISI sequences of 150 SCN neurons in hypothalamic slices. Fractal analysis, using the periodogram, Fano factor, and Allan factor, revealed the presence of a 1/f-type power-law (fractal) behavior in the ISI sequences. This fractal nature was persistent after the application of the GABAA receptor antagonist bicuculline, suggesting that the fractal stochastic activity is an intrinsic property of individual SCN neurons. Based on these physiological findings, we developed a computational model for the stochastic SCN neurons to find that their stochastic spiking activity was best described by a gamma point process whose mean firing rate was modulated by a fractal binomial noise. Taken together, we suggest that SCN neurons generate temporal spiking patterns using the fractal stochastic point process.
Similar content being viewed by others
References
Achermann P, Kunz H (1999) Modeling circadian rhythm generation in the suprachiasmatic nucleus with locally coupled self-sustained oscillators: Phase shifts and phase response curves. Journal of Biological Rhythms 14: 460–468.
Allan DW (1966) Statistics of atomic frequency standards. Proceedings of the IEEE 54: 221–230.
Antle MC, Foley DK, Foley NC, Silver R (2003) Gates and oscillators: A network model of the brain clock. Journal of Biological Rhythms 18: 339–350.
Colwell CS (2000) Rhythmic coupling among cells in the suprachiasmatic nucleus. Journal of Neurobiology 43: 379–388.
Cox DR, Lewis PAW (1966) The Statistical Analysis of Series of Events. Wiley, New York, pp. 17–36.
Forger DB, Dean DA 2nd, Gurdziel K, Leloup JC, Lee C, Von Gall C, Etchegaray JP, Kronauer RE, Goldbeter A, Peskin CS, Jewett ME, Weaver DR (2003) Development and validation of computational models for mammalian circadian oscillators. OMICS A Journal of Integrative Biology 7: 387–400.
Gillette MU (1991) SCN electrophysiology in vitro: Rhythmic activity and endogenous clock properties. In: DC Klein, RY Moore, SM Reppert, eds. Suprachiasmatic Nucleus: The Mind’s Clock, Oxford University Press, New York, NY, pp. 125–143.
Goldbeter A (1995) A model for circadian oscillations in the Drosophila period protein (PER). Proceeding of Rotal Society in Lond B Biological Science 261: 319–324.
Gruneis F, Nakao M, Mizutani Y, Yamamoto M, Meesmann M, Musha T (1993) Further study on 1/f fluctuations observed in central single neurons during REM sleep. Biological Cybernetics 68: 193–198.
Inouye ST, Kawamura H (1979) Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proceedings of National Academy of Sciences USA 76: 5962–5966.
Jagota A, de la Iglesia HO, Schwartz WJ (2000) Morning and evening circadian oscillations in the suprachiasmatic nucleus in vitro. Nature Neuroscience 3: 372–376.
Jeong J, Kwak Y, Kim YI, Lee KJ (submitted) Temporal dynamics underlying spiking patterns of the rat suprachiasmatic nucleus in vitro.
Jewett ME, Kronauer RE (1998) Refinement of a limit cycle oscillator model of the effects of light on the human circadian pacemaker. Journal of Theoretical Biology 192: 455–465. Erratum in: J Theor Biol 1998, 194: 605.
Kelly OE, Johnson DH, Delgutte B, Cariani P (1996) Fractal noise strength in auditory-nerve fiber recordings. Journal of the Acoustical Society of America 99: 2210–2220.
Kronauer RE (1990) A quantitative model for the effects of light on the amplitude and phase of the deep circadian pacemaker, based on human data. In JA Horne, ed. Sleep’90. Ponrenagel, Bocunm, Germany, pp. 306–309.
Kumar AR, Johnson DH (1993) Analyzing and modeling fractal intensity point processes. Journal of the Acoustical Society of America 93: 3365–3373.
Läuger P (1988) Internal motions in proteins and gating kinetics of ionic channels. Biophysical Journal 53: 877–884.
Leloup JC, Goldbeter A (2003) Toward a detailed computational model for the mammalian circadian clock. Proceedings of National Academy of Science in USA 100: 7051–7056.
Leloup JC, Gonze D, Goldbeter A (1999) Limit cycle models for circadian rhythms based on transcriptional regulation in Drosophila and Neurospora. Journal of Biological Rhythms 14: 433–448.
Levine MW (1980) Firing rate of a retinal neuron is not predictable from interspike interval statistics. Biophysical Journal 30: 9–25.
Lewis CD, Gerard LG, Peter DL, Susan MB (2001) Long-term correlations in the spike trains of medullary sympathetic neurons. Journal of Neurophysiology 85: 1614–1622.
Liebovitch LS, Koniarek JP (1992) Ion channel kinetics. Protein switching between conformational states is fractal in time. IEEE Engineering in Medicine and Biology 11: 53–56.
Liebovitch LS, Toth TI (1991) A model of ion channel kinetics using deterministic chaotic rather than stochastic processes. Journal of Theoretical Biology 148: 243–267.
Liebovitch LS, Toth TI (1990) Using fractals to understand the opening and closing of ion channels. Annals of Biomedical Engineering 18: 177–194.
Linkenkaer-Hansen K, Nikouline VV, Palva JM, Ilmoniemi RJ (2001) Long-range temporal correlations and scaling behavior in human brain oscillations. Journal of Neuroscience 21: 1370–1377.
Linkenkaer-Hansen K, Nikulin VV, Palva JM, Kaila K, Ilmoniemi RJ (2004) Stimulus-induced change in long-range temporal correlations and scaling behaviour of sensorimotor oscillations. European Journal of Neuroscience 19: 203–211.
Liu C, Weaver DR, Strogatz SH, Reppert SM (1997) Cellular construction of a circadian clock: Period determination in the suprachiasmatic nuclei. Cell 91:855–860.
Longtin A (1993) Nonlinear forecasting of spike trains from sensory neurons. International Journal of Bifurcation and Chaos 3: 651–661.
Lowen SB, Teich MC (1993) Fractal renewal processes. IEEE Transactions in Information Theory 39: 1669–1671.
Lowen SB, Liebovitch LS, White JA (1999) Fractal ion-channel behavior generates fractal firing patterns in neuronal models. Physical Review E 59: 5970–80.
Lowen SB, Teich MC (1996) The periodogram and Allan variance reveal fractal exponents greater than unity in auditory-nerve spike trains. Journal of the Acoustical Society of America 99: 3585–3591.
Lowen SB, Cash SS, Poo M, Teich MC (1997) Quantal neurotransmitter secretion rate exhibits fractal behavior. Journal of Neuroscience 17: 5666–5677.
Lowen SB, Ozaki T, Kaplan E, Saleh BEA, Teich MC (2001) Fractal features of dark, maintained, and driven neural discharges in the cat visual system. Methods 24: 377–394.
Marom S (1998) Slow changes in the availability of voltage-gated ion channels: Effects on the dynamics of excitable membranes. Journal of Membrane Biology 161: 105–113.
Meijer JH, Rietveld WJ (1989) Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiological Reviews 69: 671–707.
Millhauser GL, Salpeter EE, Oswald RE (1988) Diffusion models of ion-channel gating and the origin of power-law distributions from single-channel recording. Proceedings of National Academy of Science 85: 1503–1507.
Moore RY, Speh JC (1993) GABA is the principal neurotransmitter of the circadian system. Neuroscience Letters 150: 112–116.
Morin LP (1994) The circadian visual system. Brain Research Reviews 19: 102–127.
Newman GC, Hospod FE, Patlak CS, Moore RY (1992) Analysis of in vitro glucose utilization in a circadian pacemaker model. Journal of Neuroscience 12: 2015–2021.
Okamura H, Berod A, Julien JF, Geffard M, Kitahama K, Mallet J, Bobillier P (1989) Demonstration of GABAergic cell bodies in the suprachiasmatic nucleus: in situ hybridization of glutamic acid decarboxylase (GAD) mRNA and immunocytochemistry of GAD and GABA. Neuroscience Letter 102: 131–136.
Pavlidis T (1967) A model for circadian clocks. Bulletin in Mathematical Biophysics 29: 781–791.
Pennartz CM, Bierlaagh MA, Geurtsen AM (1997) Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: Involvement of a slowly inactivating component of sodium current. Journal of Neurophysiology 78: 1811–1825.
Pennartz CM, De Jeu MT, Geurtsen AM, Sluiter AA, Hermes ML (1998) Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. Journal of Physiology 506: 775–793.
Powers NL, Salvi RJ (1992) In: Abstracts of the XV Midwinter Reseach Meeting, Association for Research in Otolaryngology 292, p. 101.
Reppert SM, Weaver DR (2001) Molecular analysis of mammalian circadian rhythms. Annual Review of Physiology 63: 647–676.
Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418: 935–941.
Schaap J, Pennartz CM, Meijer JH (2003) Electrophysiology of the circadian pacemaker in mammals. Chronobiology International 20:171–188.
Schreiber T, Schmitz A (2000) Surrogate data methods. Physica D 142: 346–382.
Schwartz WJ, Gross RA, Morton MT (1987) The suprachiasmatic nuclei contain a tetrodotoxin-resistant circadian pacemaker. Proceedings of National Academy of Sciences USA 84: 1694–1698.
Shen Y, Olbrich E, Achermann P, Meier PF (2003) Dimensional complexity and spectral properties of the human sleep EEG. Clinical Neurophysiology 114: 199–209.
Shirakawa T, Honma S, Honma K (2001) Multiple oscillators in the suprachiasmatic nucleus. Chronobiology International 18: 371–387.
Soen Y, Braun E (2000) Scale-invariant fluctuations at different levels of organization in developing heart cell networks. Physical Review E 61: R2216–R2219.
Steedman WM, Zachary S (1990) Characteristics of background and evoked discharges of multireceptive neurons in lumbar spinal cord of cat. Journal of Neurophysiology 63: 1–15.
Steedman WM, Iggo A, Molony V, Korogod S, Zachary S (1983) Statistical analysis of ongoing activity of neurones in the substantia gelatinosa and in lamina III of cat spinal cord. Quarterly Journal of Experimental Physiology 68: 733–746.
Teich MC, Heneghan C, Lowen SB, Ozaki T, Kaplan E (1997) Fractal character of the neural spike train in the visual system of the cat. Journal of Optical Society of America A 14: 529–546.
Teich MC (1989) Fractal character of the auditory neural spike train. IEEE Transactions in Biomedical Engineering 36: 150–160.
Toib A, Lyakhov V, Marom S (1998) Interaction between duration of activity and time course of recovery from slow inactivation in mammalian brain Na+ channels. Journal of Neuroscience 18: 1893–1903.
Tuckwell HC (1989) Stochastic processes in the neurosciences. Society for Industrial and Applied mathematics, Philadelphia, PA.
Turcott RG, Barker PDR, Teich MC (1995) Long-duration correlation in the sequence of action potentials in an insect visual interneuron. Journal of Statistical Computation and Simulation 52: 253–271.
Van Den Pol AN, Dudek FE (1993) Cellular communication in the circadian clock, the suprachiasmatic nucleus. Neuroscience 56: 793–811.
Van Den Pol AN, Finkbeiner SM, Cornell-Bell AH (1992) Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. Journal of Neuroscience 12: 2648–2664.
West BJ (1990) Fractal Physiology and Chaos in Medicine. World Scientific, Singapore, pp.67–78.
Wever R (1972) Virtual synchronization towards the limits of the range of entrainment. Journal of Theoretical Biology 36: 119–132.
Winfree AT (2002) Oscillating systems. On emerging coherence. Science 298: 2336–2337.
Wise ME (1981) In: Statistical Distributions in Scientific Work. Reidel, Boston, pp. 211–231.
Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H (2003) Synchronization of cellular clocks in the suprachiasmatic nucleus. Science 302: 1408–1412.
Author information
Authors and Affiliations
Additional information
Action Editor: Carson C. Chow
Rights and permissions
About this article
Cite this article
Kim, SI., Jeong, J., Kwak, Y. et al. Fractal Stochastic Modeling of Spiking Activity in Suprachiasmatic Nucleus Neurons. J Comput Neurosci 19, 39–51 (2005). https://doi.org/10.1007/s10827-005-0149-x
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10827-005-0149-x