Abstract
Avian brain area HVC is known to be important for the production of birdsong. In zebra finches, each RA-projecting neuron in HVC emits a single burst of spikes during a song motif. The population of neurons is activated in a precisely timed, stereotyped sequence. We propose a model of these burst sequences that relies on two hypotheses. First, we hypothesize that the sequential order of bursting is reflected in the excitatory synaptic connections between neurons. Second, we propose that the neurons are intrinsically bursting, so that burst duration is set by cellular properties. Our model generates burst sequences similar to those observed in HVC. If intrinsic bursting is removed from the model, burst sequences can also be produced. However, they require more fine-tuning of synaptic strengths, and are therefore less robust. In our model, intrinsic bursting is caused by dendritic calcium spikes, and strong spike frequency adaptation in the soma contributes to burst termination.








Similar content being viewed by others
Notes
Note that the associative chaining model is ideally suited to the stereotypy of zebra finch song, which contrasts with the extreme diversity of sequences generated by humans. Lashley argued that a hierarchical neural representation is necessary for generating such diversity (Lashley 1951).
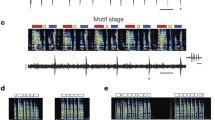
To be more accurate, about half the HVC(RA) neurons do this, while the other half are inactive (Hahnloser et al. 2002).
In the more general correlation matrix model studied by these authors, a single neuron is allowed to belong to more than one group.
This diagram is based primarily on the work of Mooney and Prather (2005). The evidence for recurrent inhibition is strong, but the excitatory interactions between projection neurons are somewhat speculative.
While these statements are applicable for an idealized model of sequence generation, a real neurobiological system might deviate somewhat from the ideal, as detailed in Section 4.
If the connection strength supports a stable propagation of single spikes, it is possible to propagate any number of spikes per neuron by inducing long spike trains at low frequency in the neurons of the first group (data not shown). Such propagation however does not agree with the observed short high frequency (about 600 Hz) bursts of spikes in HVC(RA) neurons (Hahnloser et al. 2002).
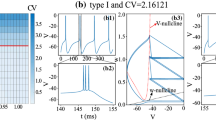
The number of spikes decreases slightly with the increase of the synaptic input to the dendrite (Fig. 6(a)). This is because the reversal potential of the calcium current (120 mV) is much larger than that of the synaptic current (0 mV). Increasing the synaptic input thus slightly decreases the strength of the calcium spike.
While Mooney and Prather reported synaptic interactions between pairs of HVC(RA) neurons in vitro (Mooney and Prather 2005), it is not clear whether these connections were monosynaptic.
Bidirectional propagation is standard for most excitable media. For example, an axon can support either orthodromic or antidromic propagation of an action potential, though only the orthodromic is seen in natural conditions.
Another ambiguity of definition arises when considering the connectivity of Fig. 3(c). In this model, the connectivity is unidirectional but the neurons are not divided into groups. Here the spike times of the neurons will not cluster into groups, but are expected to be fairly uniformly distributed in time. Nevertheless, synchronous (within a synaptic integration time) spiking may be required for propagation of activity. It is not clear whether this should be called a synfire chain.
References
Abeles, M. (1982). Local cortical circuits: An electrophysiological study (pp. 83–92). Berlin Heidelberg New York: Springer.
Abeles, M. (1991). Corticonics. Cambridge, UK: Cambridge University Press.
Amari, S. (1972). Learning patterns and pattern sequences by self-organizing nets of threshold elements. IEEE Transactions on Computers, C-21, 1197–1206.
Brainard, M. S., & Doupe, A. J. (2000). Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature, 404, 762–766.
Brumberg, J. C., Nowak, L. G., & McCormick, D. A. (2000). Ionic mechanisms underlying repetitive high-frequency burst firing in supragranular cortical neurons. Journal of Neuroscience, 20, 4829–4843.
Cardin, J. A., Raksin, J. N., & Schmidt, M. F. (2004). The sensorimotor nucleus NIf is necessary for auditory processing but not vocal motor output in the avian song system. Journal of Neurophysiology.
Cateau, H., & Fukai, T. (2001). Fokker–Planck approach to the pulse packet propagation in synfire chain. Neural Networks, 14, 675–685.
Chi, Z., & Margoliash, D. (2001). Temporal precision and temporal drift in brain and behavior of zebra finch song. Neuron, 32, 899–910.
Coleman, M. J., & Vu, E. T. (2005). Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. Journal of Neurobiology, 63, 70–89.
Crook, S. M., Ermentrout, G. B., & Bower, J. M. (1998). Spike frequency adaptation affects the synchronization properties of networks of cortical oscillations. Neural Computation, 10, 837–854.
Davis, G. W. (2006). Homeostatic control of neural activity: From phenomenology to molecular design. Annual Review of Neuroscience, 29, 307–323.
Denk, W., & Horstmann, H. (2004). Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol, 2, e329.
Diesmann, M., Gewaltig, M. O., & Aertsen, A. (1999). Stable propagation of synchronous spiking in cortical neural networks. Nature, 402, 529–533.
Dodson, P. D., Barker, M. C., & Forsythe, I. D. (2002). Two heteromeric Kv1 potassium channels differentially regulate action potential firing. Journal of Neuroscience, 22, 6953–6961.
Doupe, A. J., & Kuhl, P. K. (1999). Birdsong and human speech: Common themes and mechanisms. Annual Review of Neuroscience, 22, 567–631.
Doya, K., & Sejnowski, T. J. (1999). A computational model of avian song learning. In M. S. Gazzaniga (Ed.), The new cognitive neurosciences. Cambridge, MA: MIT Press.
Drew, P. J., & Abbott, L. F. (2003). Model of song selectivity and sequence generation in area HVc of the songbird. Journal of Neurophysiology, 89, 2697–2706.
Dutar, P., Vu, H. M., & Perkel, D. J. (1998). Multiple cell types distinguished by physiological, pharmacological, and anatomic properties in nucleus HVc of the adult zebra finch. Journal of Neurophysiology, 80, 1828–1838.
Ermentrout, B. (1998). The analysis of synaptically generated traveling waves. Journal of Computational Neuroscience, 5, 191–208.
Estes, W. K. (1972). An associative basis for coding and organisation in memory. In A. W. Melton, & E. Martin (Eds.), Coding processes in human memory. Washington, DC: Winston.
Euler, T., Detwiler, P. B., & Denk, W. (2002). Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature, 418, 845–852.
Fee, M. S., Kozhevnikov, A. A., & Hahnloser, R. H. (2004). Neural mechanisms of vocal sequence generation in the songbird. Annals of the New York Academy of Sciences, 1016, 153–170.
Fortune, E. S., & Margoliash, D. (1995). Parallel pathways and convergence onto HVc and adjacent neostriatum of adult zebra finches (Taeniopygia guttata). Journal of Comparative Neurology, 360, 413–441.
Franceschetti, S., Guatteo, E., Panzica, F., Sancini, G., Wanke, E., & Avanzini, G. (1995). Ionic mechanisms underlying burst firing in pyramidal neurons: Intracellular study in rat sensorimotor cortex. Brain Research, 696, 127–139.
Golding, N. L., Jung, H. Y., Mickus, T., & Spruston, N. (1999). Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. Journal of Neuroscience, 19, 8789–8798.
Golomb, D., & Amitai, Y. (1997). Propagating neuronal discharges in neocortical slices: Computational and experimental study. Journal of Neurophysiology, 78, 1199–1211.
Hahnloser, R. H., Kozhevnikov, A. A., & Fee, M. S. (2002). An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature, 419, 65–70.
Hahnloser, R. H., Kozhevnikov, A. A., & Fee, M. S. (2006). Sleep-related neural activity in a premotor and a basal-ganglia pathway of the songbird. Journal of Neurophysiology, 96, 794–812.
Hausser, M., Spruston, N., & Stuart, G. J. (2000). Diversity and dynamics of dendritic signaling. Science, 290, 739–744.
Hermann, M., Hertz, J. A., & Prugel-Bennet, A. (1995). Analysis of synfire chains. Network: Computation in Neural Systems, 6, 403–414.
Hodgkin, A. L. (1948). The local electric changes associated with repetitive action in a non-medulated axon. Journal of Physiology, 107, 165–181.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology (London), 117, 500–544.
Immelmann, K. (1969). Song development in the zebra finch and other estrildid finches. In R. Hinde (Ed.), Bird vocalization (pp. 61–74). Cambridge, UK: Cambridge University Press.
Katz, L. C., & Gurney, M. E. (1981). Auditory responses in the zebra finch’s motor system for song. Brain Research, 221, 192–197.
Kistler, W. M. (2000). Stability properties of solitary waves and periodic wave trains in a two-dimensional network of spiking neurons. Physical Review. E, Statistical Physics, Plasmas, Fluids, and Related Interdisciplinary Topics, 62, 8834–8837.
Kistler, W. M., & Gerstner, W. (2002). Stable propagation of activity pulses in populations of spiking neurons. Neural Computation, 14, 987–997.
Kleinfeld, D. (1986). Sequential state generation by model neural networks. Proceedings of the National Academy of Sciences of the United States of America, 83, 9469–9473.
Konishi, M. (1965). The role of auditory feedback in the control of vocalization in the white-crowned sparrow. Zeitschrift für Tierpsychologie, 22, 770–783.
Kozhevnikov, A., & Fee, M. S. (2006). Singing-related activity of identified HVC neurons in the zebra finch. Journal of Neurophysiology.
Kubota, M., & Taniguchi, I. (1998). Electrophysiological characteristics of classes of neuron in the HVc of the zebra finch. Journal of Neurophysiology, 80, 914–923.
Lashley, K. S. (1951). The problem of serial order in behavior. In L. A. Jeffress (Ed.), Cerebral mechanisms in behavior (the Hixon Symposium) (pp. 112–136). New York: Wiley.
Leonardo, A., & Fee, M. S. (2005). Ensemble coding of vocal control in birdsong. Journal of Neuroscience, 25, 652–661.
Lewicki, M. S. (1996). Intracellular characterization of song-specific neurons in the zebra finch auditory forebrain. Journal of Neuroscience, 16, 5855–5863.
Lien, C. C., & Jonas, P. (2003). Kv3 potassium conductance is necessary and kinetically optimized for high-frequency action potential generation in hippocampal interneurons. Journal of Neuroscience, 23, 2058–2068.
Manis, P. B., & Marx, S. O. (1991). Outward currents in isolated ventral cochlear nucleus neurons. Journal of Neuroscience, 11, 2865–2880.
Mattia, D., Kawasaki, H., & Avoli, M. (1997). In vitro electrophysiology of rat subicular bursting neurons. Hippocampus, 7, 48–57.
Mooney, R. (2000). Different subthreshold mechanisms underlie song selectivity in identified HVc neurons of the zebra finch. Journal of Neuroscience, 20, 5420–5436.
Mooney, R., Hoese, W., & Nowicki, S. (2001). Auditory representation of the vocal repertoire in a songbird with multiple song types. Proceedings of the National Academy of Sciences of the United States of America, 98, 12778–12783.
Mooney, R., & Prather, J. F. (2005). The HVC microcircuit: The synaptic basis for interactions between song motor and vocal plasticity pathways. Journal of Neuroscience, 25, 1952–1964.
Nixdorf, B. E., Davis, S. S., & DeVoogd, T. J. (1989). Morphology of Golgi-impregnated neurons in hyperstriatum ventralis, pars caudalis in adult male and female canaries. Journal of Comparative Neurology, 284, 337–349.
Nottebohm, F., Kelley, D. B., & Paton, J. A. (1982). Connections of vocal control nuclei in the canary telencephalon. Journal of Comparative Neurology, 207, 344–357.
Nottebohm, F., Stokes, T. M., & Leonard, C. M. (1976). Central control of song in the canary, Serinus canarius. Journal of Comparative Neurology, 165, 457–486.
Osan, R., Curtu, R., Rubin, J., & Ermentrout, B. (2004). Multiple-spike waves in a one-dimensional integrate-and-fire neural network. Journal of Mathematical Biology, 48, 243–274.
Pinsky, P. F., & Rinzel, J. (1994). Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. Journal of Computational Neuroscience, 1, 39–60.
Press, W., Teukolsky, S., Vetterling, W., & Flannery, B. (1992). Numerical recipes in C. Cambridge, UK: Cambridge University Press.
Rathouz, M., & Trussell, L. (1998). Characterization of outward currents in neurons of the avian nucleus magnocellularis. Journal of Neurophysiology, 80, 2824–2835.
Reyes, A. D. (2003). Synchrony-dependent propagation of firing rate in iteratively constructed networks in vitro. Nature Neuroscience, 6, 593–599.
Reyes, A. D., Rubel, E. W., & Spain, W. J. (1994). Membrane properties underlying the firing of neurons in the avian cochlear nucleus. Journal of Neuroscience, 14, 5352–5364.
Rinzel, J., & Ermentrout, G. B. (1989). Analysis of neural excitability and oscillations. In C. Koch, & I. Segev (Eds.), Methods in neuronal modeling. Cambridge: MIT Press.
Scharff, C., Kirn, J. R., Grossman, M., Macklis, J. D., & Nottebohm, F. (2000). Targeted neuronal death affects neuronal replacement and vocal behavior in adult songbirds. Neuron, 25, 481–492.
Schiller, J., Major, G., Koester, H. J., & Schiller, Y. (2000). NMDA spikes in basal dendrites of cortical pyramidal neurons. Nature, 404, 285–289.
Schwindt, P., & Crill, W. (1999). Mechanisms underlying burst and regular spiking evoked by dendritic depolarization in layer 5 cortical pyramidal neurons. Journal of Neurophysiology, 81, 1341–1354.
Sompolinsky, H., & Kanter, I. I. (1986). Temporal association in asymmetric neural networks. Physical Review Letters, 57, 2861–2864.
Svirskis, G., Kotak, V., Sanes, D. H., & Rinzel, J. (2002). Enhancement of signal-to-noise ratio and phase locking for small inputs by a low-threshold outward current in auditory neurons. Journal of Neuroscience, 22, 11019–11025.
Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G., Sanes, J. R., Welker, E., et al. (2002). Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature, 420, 788–794.
Traub, R. D., Jefferys, J. G., & Miles, R. (1993). Analysis of the propagation of disinhibition-induced after-discharges along the guinea-pig hippocampal slice in vitro. Journal of Physiology, 472, 267–287.
Traub, R. D., Jefferys, J. G., Miles, R., Whittington, M. A., & Toth, K. (1994). A branching dendritic model of a rodent CA3 pyramidal neurone. Journal of Physiology, 481(Pt 1), 79–95.
Troyer, T. W., & Doupe, A. J. (2000). An associational model of birdsong sensorimotor learning II. Temporal hierarchies and the learning of song sequence. Journal of Neurophysiology, 84, 1224–1239.
Tsai, P. S., Friedman, B., Ifarraguerri, A. I., Thompson, B. D., Lev-Ram, V., Schaffer, C. B., et al. (2003). All-optical histology using ultrashort laser pulses. Neuron, 39, 27–41.
Vicario, D. S. (1991). Organization of the zebra finch song control system: II. Functional organization of outputs from nucleus Robustus archistriatalis. Journal of Comparative Neurology, 309, 486–494.
Vu, E. T., Mazurek, M. E., & Kuo, Y. C. (1994). Identification of a forebrain motor programming network for the learned song of zebra finches. Journal of Neuroscience, 14, 6924–6934.
Wang, L. Y., Gan, L., Forsythe, I. D., & Kaczmarek, L. K. (1998). Contribution of the Kv3.1 potassium channel to high-frequency firing in mouse auditory neurones. Journal of Physiology, 509(Pt 1), 183–194.
Wang, X. J. (1999). Fast burst firing and short-term synaptic plasticity: A model of neocortical chattering neurons. Neuroscience, 89, 347–362.
Wei, D. S., Mei, Y. A., Bagal, A., Kao, J. P., Thompson, S. M., & Tang, C. M. (2001). Compartmentalized and binary behavior of terminal dendrites in hippocampal pyramidal neurons. Science, 293, 2272–2275.
Wild, J. M., Williams, M. N., Howie, G. J., & Mooney, R. (2005). Calcium-binding proteins define interneurons in HVC of the zebra finch (Taeniopygia guttata). Journal of Comparative Neurology, 483, 76–90.
Williams, H. (2004). Birdsong and singing behavior. Annals of the New York Academy of Sciences, 1016, 1–30.
Williams, H., & Vicario, D. S. (1993). Temporal patterning of song production: Participation of nucleus uvaeformis of the thalamus. Journal of Neurobiology, 24, 903–912.
Wong, R. K., & Stewart, M. (1992). Different firing patterns generated in dendrites and somata of CA1 pyramidal neurones in guinea-pig hippocampus. Journal of Physiology, 457, 675–687.
Yu, A. C., & Margoliash, D. (1996). Temporal hierarchical control of singing in birds. Science, 273, 1871–1875.
Acknowledgement
Research was supported by The Huck Institute of Life Sciences at the Pennsylvania State University and Alfred P. Sloan Fellowship (DZJ), and Howard Hughes Medical Institute (FR, HSS). DZJ thanks the Kavli Institute for Theoretical Physics at University of California, Santa Barbara for partial support of this work. We thank Michael Long, Anthony Leonardo and Michale Fee for useful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Alain Destexhe
Rights and permissions
About this article
Cite this article
Jin, D.Z., Ramazanoğlu, F.M. & Seung, H.S. Intrinsic bursting enhances the robustness of a neural network model of sequence generation by avian brain area HVC. J Comput Neurosci 23, 283–299 (2007). https://doi.org/10.1007/s10827-007-0032-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-007-0032-z