Abstract
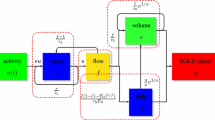
This paper extends a previously formulated deterministic metabolic/hemodynamic model for the generation of blood oxygenated level dependent (BOLD) responses to include both physiological and observation stochastic components (sMHM). This adds a degree of flexibility when fitting the model to actual data by accounting for un-modelled activity. We then show how the innovation method can be used to estimate unobserved metabolic/hemodynamic as well as vascular variables of the sMHM, from simulated and actual BOLD data. The proposed estimation method allowed for doing model comparison by calculating the model’s AIC and BIC. This methodology was then used to select between different neurovascular coupling assumptions underlying sMHM. The proposed framework was first validated on simulations and then applied to BOLD data from a motor task experiment. The models under comparison in the analysis of the actual data considered that vascular response was coupled to: (I) inhibition, (II) excitation, (III) both excitation and inhibition. Data was best described by model II, although model III was also supported.










Similar content being viewed by others
References
Abeles, M. (1991). Corticonics: Neural circuits of the cerebral cortex. Cambridge: Cambridge University Press.
Aitchison, J., & Dunsmore, I. R. (1975). Statistical prediction analysis. Cambridge, UK: Cambridge University Press.
Akaike, (1973). Information theory and an extension of the maximum likelihood principle. In B. N. Petrox, & F. Caski (Eds.), Second International Symposium on Information Theory (p. 267). Budapest: Akademiai Kiado.
Akaike, (1983). Information measures and model selection. Bulletin of the International Statistical Bulletin, 50, 277–290.
Attwell, D., & Iadecola, C. (2002). The neural basis of functional brain imaging signals. Trends in Neurosciences, 25, 621–625.
Biswal, B., Yetkin, F. Z., Haughton, V. M., & Hyde, J. S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34, 537–541.
Buxton, R. B., Uludag, K., Dubowitz, D. J., & Liu, T. T. (2004). Modeling the hemodynamic response to brain activation. NeuroImage, 23, S220–S223.
Buxton, R. B., Wong, E. C., & Frank, L. R. (1998). Dynamics of blood flow and oxygenation changes during brain activation: The Balloon model. Medical Risk Management, 39, 855–864.
Carbonell, F., Biscay, R. J., Jímenez, J. C., & de la Cruz, H. (2007). Numerical simulation of nonlinear dynamical systems driven by commutative noise. Journal of Computational Physics, 226, 1219–1233.
Ceballos-Baumann, A. O. (2003). Functional imaging in Parkinson’s disease: Activation studies with PET, fMRI and SPECT. Journal of Neurology, 250, I15–I23.
Eke, A., & Hermán, P. (1999). Fractal analysis of spontaneous fluctuations in human cerebral hemoglobin content and its oxygenation level recorded by NIRS. Advance in Experimental Medicine and Biology, 47, 49–55.
Elwell, C. E., Springett, R., Hillmann, E., & Delpy, D. T. (1999). Oscillations in cerebral haemodynamics. Implications for functional activation studies. Advance in Experimental Medicine and Biology, 471, 57–65.
Escola, L., Michelet, T., Macia, F., Guehl, D., Bioulac, B., & Burbaud, P. (2003). Disruption of information processing in the supplementary motor area of the MPTP-treated monkey. A clue to the pathophysiology of akinesia? Brain, 126, 95–114.
Fergus, A., & Lee, K. S. (1997). GABAergic regulation of cerebral micro-vascular tone in the rat. Journal of Cerebral Blood Flow and Metabolism, 17, 992–1003.
Friston, K. J. (2002). Bayesian estimation of dynamical systems: An application to fMRI. NeuroImage, 16, 513–530.
Friston, K. J., Harrison, L., & Penny, W. (2003). Dynamic causal modeling. NeuroImage, 19, 1273–1302.
Friston, K. J., Mechelli, A., Turner, R., & Price, C. J. (2000). Nonlinear responses in fMRI: the Balloon model, Volterra kernels, and other hemodynamics. NeuroImage, 12, 466–477.
Friston, K. J., Trujillo-Barreto, N. J., & Daunizeau, J. (2008). DEM: A variational treatment of dynamic systems. NeuroImage, 41, 849–885.
Gill, P. E., Murray, W., & Wright, M. H. (1981). Practical optimization. London: Academic.
Haslinger, B., Erhard, P., Kampfe, N., Boecker, H., Rummeny, E., & Schwaiger, M. (2001). Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain, 124, 558–570.
Hermán, P., & Eke, A. (2006). Nonlinear analysis of blood cell flux fluctuations in the rat brain cortex during stepwise hypotension challenge. Journal of Cerebral Blood Flow and Metabolism, 26, 1189–1197.
Hochbruck, M., & Lubich, C. (1997). On Krylov subspace approximations to the matrix exponential operator. SIAM Journal on Numerical Analysis, 34, 1911–1925.
Hoge, R. D., Atkinson, J., Gill, B., Crelier, G. R., Marrett, S., & Pike, G. B. (1999). Stimulus-dependent BOLD and perfusion dynamics in human V1. NeuroImage, 9, 573–585.
Hu, Y., & Wilson, G. S. (1997). Rapid changes in local extracellular brain rat brain glucose observed with an in vivo glucose sensor. Journal of Neurochemistry, 68, 1745–1752.
Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nature Rev. Neuroscience, 5, 347–360.
Jiménez, J. C. (2002). A simple algebraic expression to evaluate the local linearization schemes for stochastic differential equations. Applied Mathematics Letters, 15, 775–780.
Jiménez, J. C., Biscay, R., & Ozaki, T. (2006). Inference methods for discretely observed continuous-time stochastic volatility models: A commented overview. Asian-Pacific Financial Markets, 12, 109–141.
Jiménez, J. C., & Ozaki, T. (2003). Local linearization filters for non-linear continuous-discrete state space models with multiplicative noise. International Journal of Control, 76, 1159–1170.
Jiménez, J. C., & Ozaki, T. (2006). An approximate innovtion method for the estimation of diffusion processes from discrete data. Journal of Time Series Analysis, 27, 77–97.
Jiménez, J. C., Shoji, I., & Ozaki, T. (1999). Simulation of stochastic differential equations through the Local Linearization method. A comparative study. Journal Statistical Physics, 94, 587–602.
Kass, R. E., Raftery, A. E. (1993). Bayes factors and model uncertainty. Technical Report, 254, University of Washington.
Katz, R. W. (1981). On some criteria for estimating the order of a Markov chain. Technometrics, 23, 243–249.
Kida, I., Rothman, D., & Hyder, F. (2007). Dynamics of changes in blood flow, volume and oxygenation: Implications for dynamic functional magnetic resonance imaging calibration. Journal of Cerebral Blood Flow and Metabolism, 27, 690–696.
Kong, Y., Zheng, Y., Johnston, D., Martindale, J., Jones, M., Billings, S., & Mayhew, J. (2004). A model of the dynamic relationship between blood flow and volume changes during brain activation. Journal of Cerebral Blood Flow and Metabolism, 24, 1382–1392.
Krüger, G., & Glover, G. H. (2001). Physiological noise in oxygenation-sensitive magnetic resonance imaging. Magnetic Resonance in Medicine, 46, 631–637.
Lauritzen, M. (2005). Reading vascular changes in brain imaging: Is dendritic calcium the key? National Reviews Neuroscience, 6, 77–85.
Lilliefors, H. W. (1967). On the Komogorov–Smirnov test for normality with mean and variance unknown. Journal of the American Statistical Association, 62, 399–402.
Lu, H., Golay, X., Pekar, J. J., & van Zijl, P. C. M. (2004). Sustained post-stimulus elevation in cerebral oxygen utilization after vascular recovery. Journal of Cerebral Blood Flow and Metabolism, 24, 764–770.
Mattay, V. S., Tessitore, A., Callicott, A., Bertolino, A., Goldberg, T. E., & Chase, T. (2002). Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Annals of Neurology, 51, 156–164.
Mandeville, J. B., Marota, J. J., Ayata, C., Zararchuk, G., Moskowitz, M. A., & Rosen, B. (1999). Evidence of a cerebrovascular postarteriole windkessel with delayed compliance. Journal of Cerebral Blood Flow and Metabolism, 19, 679–689.
MacKay, D. J. C. (1992). Bayesian interpolation. Neural Computation, 4, 415–447.
McNay, E. C., & Gold, P. E. (2002). Food for thought: fluctuations in brain extracellular glucose provide insight into the mechanisms of memory modulation. Behavioural and Cognitive Neuroscience Reviews, 1, 264–280.
Miyachi, S., Lu, X., Imanishi, M., Sawada, K., Nambu, A., & Takada, M. (2006). Somatotopically arranged inputs from putamen and subthalamic nucleus to primary motor cortex. Neuroscience Research, 56, 300–308.
Nambu, A. (2005). A new approach to understand the pathophysiology of Parkinson’s disease. Journal of Neurology, 252(Suppl 4), IV/1–IV/4.
Nolsoe, K., Nielsen, J. N., & Madsen, H. (2000). Prediction-based estimating function for diffusion processes with measurement noise. Technical Reports, No. 10, Informatics and Mathematical Modelling, Technical University of Denmark.
Ozaki, T. (1994). The local linearization filter with application to nonlinear system identifications. In h. Bozdogan (Ed.), Proceedings of the first US/Japan conference on the frontiers of statistical modeling: an informational approach (pp. 217–240). Dordrecht: Kluwer Academic.
Pedroso, L. M., Marrero, A., de Arazoza, H. (2003). Nonlinear parametric model identification using genetic algorithms. Lecture Notes in Computer Science 2687 (473–480). Springer, Heidelberg.
Pellerin, L., & Magistretti, P. J. (1994). Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proceedings of the National Academy of Sciences of the United States of America, 91, 10625–10629.
Penny, W., Flandin, G., & Trujillo-Barreto, N. (2007). Bayesian comparison of spatially regularised general linear models. Human Brain Mapping, 28, 275–293.
Penny, W. D., Stephan, K. E., Mechelli, A., & Friston, K. J. (2004). Comparing dynamic causal models. NeuroImage, 22, 1157–1172.
Riera, J., Jimenez, J. C., Wan, X., Kawashima, R., & Ozaki, T. (2007). Nonlinear local electro-vascular coupling. Part II: From data to neuronal masses. Human Brain Mapping, 28, 335–354.
Riera, J., Watanabe, J., Kazuki, I., Naoki, M., Aubert, E., Ozaki, T., & Kawashima, R. (2004). A state-space model of the hemodynamic approach: Non-linear filtering of BOLD signals. NeuroImage, 21, 547–567.
Rodriguez-Rojas, R., Alvarez, L., Palmero, R., Alvarez, M., Carballo-Barreda, M., & Macias, R. (2005). Neural activity changes in the supplementary motor area induced by dopaminergic treatment in parkinsonian patients. Neurocomputing, 65, 741–749.
Sabatini, U., Boulanouar, K., Fabre, N., Martin, F., Carel, C., & Colonnese, C. (2000). Cortical motor reorganization in akinetic patients with Parkinson’s disease. Brain, 123, 394–403.
Schroeter, M., Schmiedel, O., & von Cramon, D. (2004). Spontaneous low-frequency oscillations decline in the aging brain. Journal of Cerebral Blood Flow and Metabolism, 24, 1183–1191.
Schwarz, G. (1978). Estimating the dimension of a model. Annals of Statistics, 6, 461–464.
Schweppe, F. (1965). Evaluation of likelihood function for Gaussian signals. IEEE Transactions on Information Theory, 11, 61–70.
Shibata, R. (1976). Selection of the order of an autoregressive model by Akaikes’s information criterion. Biometrika, 63, 117–126.
Shulman, R. G., Hyder, F., & Rothman, D. L. (2001). Cerebral energetics and the glycogen shunt: Neurochemical basis of functional imaging. Proceedings of the National Academy of Sciences of the United States of America, 98, 6417–6422.
Solo, V. (1980). Some aspects of recursive parameter estimation. International Journal of Control, 32, 395–410.
Sotero, R. C., & Trujillo-Barreto, N. J. (2007). Modelling the role of excitatory and inhibitory neuronal activity in the generation of the BOLD signal. NeuroImage, 35, 149–165.
Talairach, J., & Tournoux, P. (1988). A co-planar stereotaxic atlas of a human brain. Stuttgart: Thieme.
Tamura, H., Kaneko, H., Kawasaki, K., & Fujita, I. (2004). Presumed inhibitory neurons in the macaque inferior temporal cortex: Visual response properties and functional interactions with adjacent neurons. Journal of Neurophysiology, 91, 2782–2796.
Valabrègue, R., Aubert, A., Burger, J., Bittoun, J., & Costalat, R. (2003). Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. Journal of Cerebral Blood Flow and Metabolism, 23, 536–545.
Valdés, P., Jimenez, J. C., Riera, J., Biscay, R., & Ozaki, T. (1999). Nonlinear EEG analysis based on a neural mass model. Biological Cybernetics, 81, 415–424.
Worsley, K. J., Liao, C., Aston, J., Petre, V., Duncan, G. H., Morales, F., & Evans, A. C. (2002). A general statistical analysis for fMRI data. NeuroImage, 15(1), 15.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Barry J. Richmond
Appendices
Appendix A: Expression for the drift
The drift term of system (8) is given by:
where \(\lambda = 2 - \frac{1}{{1 - e^{\theta _5 \theta _6 } }}\)
Appendix B: Local linearization filter algorithm
Let a state-space model be defined by the continuous state equation:
and the discrete observation equation:
Assuming that θ is given and starting with \(\widehat{\mathbf{y}}_{{{t_0 } \mathord{\left/ {\vphantom {{t_0 } {t_0 }}} \right. \kern-\nulldelimiterspace} {t_0 }}} = {\mathbf{y}}_{{{t_0 } \mathord{\left/ {\vphantom {{t_0 } {t_0 }}} \right. \kern-\nulldelimiterspace} {t_0 }}} \), \(\widehat{{\mathbf{P}}}_{{{t_{0} } \mathord{\left/ {\vphantom {{t_{0} } {t_{0} }}} \right. \kern-\nulldelimiterspace} {t_{0} }}} = {\mathbf{P}}_{{{t_{0} } \mathord{\left/ {\vphantom {{t_{0} } {t_{0} }}} \right. \kern-\nulldelimiterspace} {t_{0} }}} \), the LL filter for the model (20)–(21) is defined by the recursive computation of the following estimates (Jiménez and Ozaki 2003; Riera et al. 2007):
For all k = 0,...,M − 1.
-
1.
Prediction: For j = 0,…,M − 1,(M ≥ 1):
$$s_j = t_k + j\frac{{t_{k + 1} - t_k }}{M}$$(22)$$\widehat{\mathbf{y}}_{s_{j + 1} /t_k } = \widehat{\mathbf{y}}_{s_j /t_k } + \int\limits_0^{s_{j + 1} - s_j } {e^{{\mathbf{D}}_{s_j } \left( {s_{j + 1} - s_j - s} \right)} } \left( {{\mathbf{a}}\left( {s_j ,\widehat{\mathbf{y}}_{s_j /t_k } ,{\mathbf{\theta }}} \right) + {\mathbf{d}}_{s_j } s} \right)ds$$(23)$$\widehat{\mathbf{P}}_{s_{j + 1} /t_k } = e^{{\mathbf{D}}_{s_j } \left( {s_{j + 1} - s_j - s} \right)} \widehat{\mathbf{P}}_{s_j /t_k } e^{{\mathbf{D}}_{_{s_j } }^T \left( {s_{j + 1} - s_j - s} \right)} + \int\limits_0^{s_{j + 1} - s_j } {e^{{\mathbf{D}}_{s_j } s} } {\mathbf{bb}}^{\text{T}} e^{{\mathbf{D}}_{{\text{s}}_{\text{j}} }^{\text{T}} s} ds$$(24) -
2.
Innovation:
$$\widehat\vartheta _{t_{k + 1} } = z_{t_{k + 1} } - {\mathbf{c}}^T \widehat{\mathbf{y}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right.\kern-\nulldelimiterspace} {t_k }}} $$(25)$$\widehat{\text{ $ \Sigma $ }}_{t_{k + 1} /t_k }^\nu = {\mathbf{c}}^T \widehat{\mathbf{P}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right.\kern-\nulldelimiterspace} {t_k }}} {\mathbf{c}} + \theta _8 $$(26) -
3.
Filter:
$$\widehat{\mathbf{y}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_{k + 1} }}} \right.\kern-\nulldelimiterspace} {t_{k + 1} }}} = \widehat{\mathbf{y}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right.\kern-\nulldelimiterspace} {t_k }}} + {\mathbf{k}}_{t_{k + 1} } \widehat\vartheta _{t_{k + 1} } $$(27)$$\widehat{\mathbf{P}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_{k + 1} }}} \right.\kern-\nulldelimiterspace} {t_{k + 1} }}} = \widehat{\mathbf{P}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right.\kern-\nulldelimiterspace} {t_k }}} - {\mathbf{k}}_{t_{k + 1} } {\mathbf{c}}^T \widehat{\mathbf{P}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right.\kern-\nulldelimiterspace} {t_k }}} $$(28)where \({\mathbf{k}}_{t_{k + 1} } = {\mathbf{\hat P}}_{t_{k + 1} /t_k } {\mathbf{c}}^{\text{T}} \left( {\hat \Sigma _{t_{k + 1} }^\vartheta } \right)^{ - 1} \) is the filter gain. In the above algorithm M = 1 denotes the number of points between a pair of consecutive observations t k ,t k + 1 used to compute the predictions \(\widehat{\mathbf{y}}_{{{t_{k + 1} } \mathord{\left/ {\vphantom {{t_{k + 1} } {t_k }}} \right. \kern-\nulldelimiterspace} {t_k }}} \) and \(\widehat{\mathbf{P}}_{{{t_{k + 1} } \mathord{\left/ {\vphantom {{t_{k + 1} } {t_k }}} \right. \kern-\nulldelimiterspace} {t_k }}} \), \({\mathbf{D}}_{s_j } = \frac{\partial }{{\partial {\mathbf{y}}}}{\mathbf{a}}\left( {s_j ,{\mathbf{y}}_{s_j /t_k } ;{\mathbf{\theta }}} \right)\) and \({\mathbf{d}}_{s_j } = \frac{\partial }{{\partial s}}{\mathbf{a}}\left( {s_j ,{\mathbf{y}}_{s_j /t_k } ,{\mathbf{\theta }}} \right)\). Explicit expressions in terms of exponential matrices for integrals (23) and (24) are given in Jiménez and Ozaki, (2003). Thus, the numerical computation of the conditional moments \({\mathbf{\hat y}}_{t_{k + 1} /t_k } \) and \( {\mathbf{\hat P}}_{{{t_{k + 1} } \mathord{\left/{\vphantom {{t_{k + 1} } {t_k }}} \right. \kern-\nulldelimiterspace} {t_k }}}\) is reduced to use a convenient algorithm to compute matrix exponentials. This was achieved in the present paper by using Krylov subspace methods (Hochbruck and Lubich 1997).
Rights and permissions
About this article
Cite this article
Sotero, R.C., Trujillo-Barreto, N.J., Jiménez, J.C. et al. Identification and comparison of stochastic metabolic/hemodynamic models (sMHM) for the generation of the BOLD signal. J Comput Neurosci 26, 251–269 (2009). https://doi.org/10.1007/s10827-008-0109-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-008-0109-3