Abstract
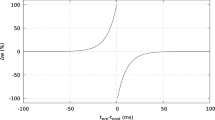
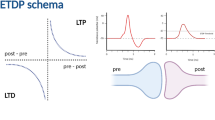
Synaptic strength can be modified by the relative timing of pre- and postsynaptic activity, a phenomenon termed spike timing-dependent plasticity (STDP). Studies of neurons in the hippocampus and in other regions have found that when presynaptic activity occurs within a narrow time window, typically 10 or 20 ms, before postsynaptic activity, long-term potentiation (LTP) is induced, while if presynaptic activity occurs within a similar time window after postsynaptic activity, long-term depression (LTD) results. The mechanisms underlying these modifications are not completely understood, although there is strong evidence that the postsynaptic Ca 2 + concentration plays a central role. Some previous modeling of STDP has focused on the dynamics of the postsynaptic Ca 2 + concentration, while other work has studied biophysical mechanisms of how a synapse can exist in, and switch between, different states corresponding to LTP and LTD. Building on previous work in these two areas we have developed the first low level STDP model of a tristable biochemical system that incorporates induction and maintenance of both LTP and LTD. Our model is able to explain the STDP observed in hippocampal neurons in response to pre- and postsynaptic pulse pairs, using only parameters derived from previous work and without the need for parameter fine-tuning. Our results also give insight into how and why the time course of the postsynaptic Ca 2 + concentration can lead to either LTP or LTD, and suggest that voltage dependent calcium channels play a key role.







Similar content being viewed by others
References
Abarbanel, H. D. I., Gibb, L., Huerta, R., & Rabinovich, M. I. (2003). Biophysical model of synaptic plasticity dynamics. Biological Cybernetics, 89, 214–226.
Abraham, W. C., & Williams, J. M. (2003). Properties and mechanisms of LTP maintenance. Neuroscientist, 9, 463–474.
Barria, A., Muller, D., Derkach, V., Griffith, L. C., & Soderling, T. R. (1997). Regulatory phosphorylation of AMPA-type glutamate receptors by CaMKII during long-term potentiation. Science, 276, 2042–2045.
Bender, V. A., Bender, K. J., Brasier, D. J., & Feldman, D. E. (2006). Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. Journal of Neuroscience, 26, 4166–4177.
Bhalla, U. S., & Iyengar, R. (1999). Emergent properties of networks of biological signaling pathways. Science, 283, 381–387.
Bi, G. Q., & Poo, G. (1998). Synaptic modifications in cultured hippocampal neurons: Dependence on spike timing, synaptic strength, and postsynaptic cell type. Journal of Neuroscience, 18, 10464–10472.
Bolshakov, V. Y., & Siegelbaum, S. A. (1994). Postsynaptic induction and presynaptic expression of hippocampal long-term depression. Science, 264, 1148–1152.
Bredt, D. S., & Nicoll, R. A. (2003). AMPA receptor trafficking at excitatory synapses. Neuron, 40, 361–379.
Carmignoto, G., & Vicini, S. (1992). Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science, 258, 1007–1011.
Caporale, N., & Dan, Y. (2008). Spike timing-dependent plasticity: A hebbian learning rule. Annual Review of Neuroscience, 31, 25–46.
Castellani, G. C., Bazzani, A., & Cooper, L. N. (2009). Toward a microscopic model of bidirectional synaptic plasticity. Proceedings of the National Academy of Science of the United States of America, 106, 14091–14095.
Castellani, G. C., Quinlan, E. M., Bersani, F., Cooper, L. N., & Shouval, H. Z. (2005). A model of bidirectional synaptic plasticity: From signaling network to channel conductance. Learning and Memory, 12, 423–432.
Castellani, G. C., Quinlan, E. M., Cooper, L. N., & Shouval, H. Z. (2001). A biophysical model of bidirectional synaptic plasticity: Dependence on AMPA and NMDA receptors. Proceedings of the National Academy of Science of the United States of America, 98, 12772–12777.
Dalby, N. O., & Mody, I. (2003). Activation of NMDA receptors in rat dentate gyrus granule cells by spontaneous and evoked transmitter release. Journal of Neurophysiology, 90, 786–797.
Froemke, R. C., Poo, M. M., Dan, Y. (2005). Spike-timing-dependent synaptic plasticity depends on dendritic location. Nature, 434, 221–225.
Gerstner, W., Kempter, R., Van Hemmen, J. L., & Wagner, H. (1996). A neuronal learning rule for sub-millisecond temporal coding. Nature, 383, 76–78.
Giordano, N. J., & Nakanishi, H. (2005). Computational physics (2nd Ed.). Saddle River: Prentice-Hall.
Graupner, M., & Brunel, N. (2007). STDP in bistable synapse model based on CaMKII and associated signaling pathways. PloS Computational Biology, 3, 2299–2323.
Hartley, M., Taylor, N., & Taylor, J. (2006). Understanding spike-time-dependent plasticity: A biologically motivated computational model. Neurocomputing, 69, 2005–2016.
Hebb, D. O. (1949). The organization of behavior. New York: Wiley.
Jahr, C. E., & Stevens, C. F. (1990). Voltage dependence of NMDA-activated macroscopic conductances predicted by single-channel kinetics. Journal of Neuroscience, 10(9), 3178–3182.
Karmarkar, U. R., & Buonomano, D. V. (2002). A model of spike-timing dependent plasticity: One or two coincidence detectors? Journal of Neurophysiology, 88, 507–513.
Kelso, S. R., Ganong, A. H., & Brown, T. H. (1986). Hebbian synapses in hippocampus. Proceedings of the National Academy of Science of the United States of America, 83, 5326–5330.
Lee, S. H., Liu, L., Wang, Y. T., & Sheng, M. (2002). Clathrin adaptor AP2 and NSF interact with overlapping sites of GluR2 and play distinct roles in AMPA receptor trafficking and hippocampal LTD. Neuron, 36, 661–674.
Lee, S. R., Escobedo-Lozoya, Y., Szatmari, E. M., & Yasuda, R. (2009). Activation of CaMKII in single dendritic spines during long-term potentiation. Nature, 458, 299–306.
Lin, J. W., Ju, W., Foster, K., Lee, S. H., Ahmadian, G., Wyszynski, M., et al. (2000). Distinct molecular mechanisms and divergent endocytotic pathways of AMPA receptor internalization. Nature Neuroscience, 3, 1282–1290.
Linden, D.J. (1997). Long-term potentiation of glial synaptic currents in cerebellar culture. Neuron, 18, 983–994.
Lisman, J., & Spruston, N. (2005). Postsynaptic depolarization requirements for LTP and LTD: A critique of spike timing-dependent plasticity. Nature Neuroscience, 8, 839–841.
Luthi, A., Chittajallu, R., Duprat, F., Palmer, M. J., Benke, T. A., Kidd, F. L., et al. (1999). Hippocampal LTD expression involves a pool of AMPARs regulated by the NSF-GluR2 interaction. Neuron, 24, 389–399.
Malenka, R. C., & Nicoll, R. A. (1999). Long-term potentiationa decade of progress? Science, 285, 1870–1874.
Malinow, R., & Malenka, R. C. (2002). AMPA receptor trafficking and synaptic plasticity. Annual Review of Neuroscience, 25, 103–126.
Malinow, R., & Miller, J. P. (1986). Postsynaptic hyperpolarization reversibly blocks induction of long-term potentiation. Nature, 320, 529–530.
Man, H. Y., Lin, J. W., Ju, W. H., Ahmadian, G., Liu, L., Becker, L. E., et al. (2000). Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron, 25, 649–662.
Markram, H., Helm, P. J., & Sakmann, B. (1995). Dendritic calcium transients evoked by single back-propagating action potentials in rat neocortical pyramidal neurons. Journal of Physiology, 485, 1–20.
Markram, H., Lubke, J., Frotscher, M., & Sakmann, B. (1997). Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science, 275, 213–215.
Nevian, T., & Sakmann, B. (2006). Spine Ca2+ signaling in spike-timing-dependent plasticity. Journal of Neuroscience, 26, 11001–11013.
Nicoll and Malenka(1995). Contrasting properties of two forms of long-term potentiation in the hippocampus. Nature, 377, 115–118.
Pfister, J. P., & Gerstner, W. (2006). Triplets of spikes in a model of spike timing-dependent plasticity. Journal of Neuroscience, 26, 9673–9682.
Pi, H. J., & Lisman, J. E. (2008) Coupled phosphatase and kinase switches produced the tristability required for long-term potentiation and long-term depression. Journal of Neuroscience, 28, 13132–13138.
Pittenger, C., & Kandel, E. R. (2003). In search of general mechanisms for long-lasting plasticity: Aplysia and the hippocampus. Philosophical Transactions of the Royal Society of London B Biological Sciences, 358, 757–763.
Rubin, J. E., Gerkin, R. C., Bi, G. Q., & Chow, C. C. (2005). Calcium time course as a signal for spike timing-dependent plasticity. Journal of Neurophysiology, 93, 2600–2613.
Sabatini, B. L., Oertner, T. G., & Svoboda, K. (2002). The life cycle of Ca2 + ions in dendritic spines. Neuron, 33, 439–452.
Sajikumar, S., & Frey, J. U. (2003). Anisomycin inhibits the late maintenance of long-term depression in rat hippocampal slices in vitro. Neuroscience Letters, 338, 147–150.
Shi, S. H., Hayashi, Y., Petralia, R. S., Zaman, S. H., Wenthold, R. J., Svoboda, K., et al.(1999). Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science, 284, 1811–1816.
Shouval, H. Z., Bear, M. F., & Cooper, L. N. (2002). A unified model of NMDA receptor-dependent bidirectional synaptic plasticity. Proceedings of the National Academy of Science of the United States of America, 99, 10831–10836.
Sjostrom, P. J., Turrigiano, G. G., & Nelson, S. B. (2003). Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron, 39, 641–654.
Song, S., Miller, K. D., & Abbott, L. F. (2000). Competitive hebbian learning through spike-timing dependent synaptic plasticity. Nature Neuroscience, 3, 919–926.
Thiels, E., Norman, E. D., Barrionuevo, G., & Klann, E. (1998). Transient and persistent increases in protein phosphatase activity during long-term depression in the adult hippocampus in vivo. Neuroscience, 86, 1023–1029.
Urakubo, H., Honda, M., Froemke, R. C., & Kuroda, S. (2008). Requirement of an allosteric kinetics of NMDA receptors for spike timing-dependent plasticity. Journal of Neuroscience, 28, 3310–3323.
Van Rossum, M. C. W., Bi, G. Q., & Turrigiano, G. G. (2000). Stable hebbian learning from spike timing dependent plasticity. Journal of Neuroscience, 20, 8812–8821.
Wang, H. X., Gerkin, R. C., Nauen, D. W., & Bi, G. Q. (2005). Coactivation and timing-dependent integration of synaptic potentiation and depression. Nature Neuroscience, 8, 187–194.
Wolf, J. A., Moyer, J. T., & Finkel, L. H. (2005). The role of NMDA currents in state transitions of the nucleus accumbens medium spiny neuron. Neurocomputing, 65–66, 565–570.
Yuste, R., & Bonhoeffer, T. (2001). Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annual Review of Neuroscience, 24, 1071–1089.
Zhuo, M., Zhang, W., Son, H., Mansuy, I., Sobel, R. A., Seidman, J., et al. (1999). A selective role of calcineurin aalpha in synaptic depotentiation in hippocampus. Proceedings of the National Academy of Science of the United States of America, 96, 4650–4655.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: J. Rinzel
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carlson, K.D., Giordano, N. Interplay of the magnitude and time-course of postsynaptic Ca2 + concentration in producing spike timing-dependent plasticity. J Comput Neurosci 30, 747–758 (2011). https://doi.org/10.1007/s10827-010-0290-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-010-0290-z