Abstract
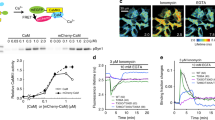
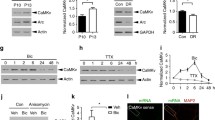
The calcium calmodulin dependent kinase (CaMKII) is important for long-term potentiation at dendritic spines. Photo-activatable GFP (PaGFP) – CaMKII fusions were used to map CaMKII movements between and within spines in dissociated hippocampal neurons. Photo-activated PaGFP (GFP*) generated in the shaft spread uniformly, but was retained for about 1 s in spines. The differential localization of GFP*-CaMKII isoforms was visualized with hundred nanometer precision frame to frame using de-noising algorithms. GFP*-CaMKIIα localized to the tips of mushroom spines. The spatiotemporal profiles of native and kinase defective GFP*-CaMKIIβ, differed markedly from GFP*-CaMKIIα and mutant GFP*-CaMKIIβ lacking the association domain. CaMKIIβ bound to cortical actin in the dendrite and the stable actin network in spine bodies. Glutamate produced a transiently localized GFP*-CaMKIIα fraction and a soluble GFP*-CaMKIIβ fraction in spine bodies. Single molecule simulations of the interplay between diffusion and biochemistry of GFP* species were guided by the spatiotemporal maps and set limits on binding parameters. They highlighted the role of spine morphology in modulating bound CaMKII lifetimes. The long residence times of GFP*-CaMKIIβ relative to GFP*-CaMKIIα followed as consequence of more binding sites on the actin cytoskeleton than the post-synaptic density. These factors combined to retain CaMKII for tens of seconds, sufficient to outlast the calcium transients triggered by glutamate, without invoking complex biochemistry.










Similar content being viewed by others
References
Andrews, S. S., Addy, N. J., Brent, R., & Arkin, A. P. (2010). Detailed simulations of cell biology with Smoldyn 2.1. PLoS Computational Biology, 6, e1000705.
Bayer, K. U., LeBel, E., McDonald, G. L., O’Leary, H., Schulman, H., & De Koninck, P. (2006). Transition from reversible to persistent binding of CaMKII to postsynaptic sites and NR2B. Journal of Neuroscience, 26, 1164–1174.
Bloodgood, B. L., & Sabatini, B. L. (2005). Neuronal activity regulates diffusion across the neck of dendritic spines. Science, 310, 866–869.
Bloodgood, B. L., & Sabatini, B. L. (2007). Ca(2+) signaling in dendritic spines. Current Opinion in Neurobiology, 17, 345–351.
Boulanger, J., Kervrann, C., Bouthemy, P., Elbau, P., Sibarita, J. B., & Salamero, J. (2010). Patch-based nonlocal functional for denoising fluorescence microscopy image sequences. IEEE Transactions on Medical Imaging, 29, 442–454.
Bourne, J., & Harris, K. M. (2007). Do thin spines learn to be mushroom spines that remember? Current Opinion in Neurobiology, 17, 381–386.
Bourne, J. N., & Harris, K. M. (2008). Balancing structure and function at hippocampal dendritic spines. Annual Review of Neuroscience, 31, 47–67.
Byrne, M.J., Waxham, M.N., Kubota, Y., (2011). The impacts of geometry and binding on CaMKII diffusion and retention in dendritic spines. Journal of Computational Neuroscience.
Cingolani, L. A., & Goda, Y. (2008). Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nature Reviews. Neuroscience, 9, 344–356.
Collins, T. J. (2007). ImageJ for microscopy. Biotechniques, 43, 25–30.
De Decker, A., Lee, J. A., & Verleysen, M. (2010). A principled approach to image denosing with similarity kernels involving patches. Neurocomputing, 73, 1198–1209.
Denk, W., Yuste, R., Svoboda, K., & Tank, D. W. (1996). Imaging calcium dynamics in dendritic spines. Current Opinion in Neurobiology, 6, 372–378.
Grant, P. A., Best, S. L., Sanmugalingam, N., Alessio, R., Jama, A. M., & Torok, K. (2008). A two-state model for Ca2+/CaM-dependent protein kinase II (alphaCaMKII) in response to persistent Ca2+ stimulation in hippocampal neurons. Cell Calcium, 44, 465–478.
Honkura, N., Matsuzaki, M., Noguchi, J., Ellis-Davies, G. C., & Kasai, H. (2008). The subspine organization of actin fibers regulates the structure and plasticity of dendritic spines. Neuron, 57, 719–729.
Huang, B., Bates, M., & Zhuang, X. (2009). Super-resolution fluorescence microscopy. Annual Review of Biochemistry, 78, 993–1016.
Hudmon, A., Lebel, E., Roy, H., Sik, A., Schulman, H., Waxham, M. N., & De Koninck, P. (2005). A mechanism for Ca2+/calmodulin-dependent protein kinase II clustering at synaptic and nonsynaptic sites based on self-association. Journal of Neuroscience, 25, 6971–6983.
Kaech, S., Brinkhaus, H., & Matus, A. (1999). Volatile anesthetics block actin-based motility in dendritic spines. Proceedings of the National Academy of Sciences of the United States of America, 96, 10433–10437.
Kaech, S., Parmar, H., Roelandse, M., Bornmann, C., & Matus, A. (2001). Cytoskeletal microdifferentiation: a mechanism for organizing morphological plasticity in dendrites. Proceedings of the National Academy of Sciences of the United States of America, 98, 7086–7092.
Kanaseki, T., Ikeuchi, Y., Sugiura, H., & Yamauchi, T. (1991). Structural features of Ca2+/calmodulin-dependent protein kinase II revealed by electron microscopy. The Journal of Cell Biology, 115, 1049–1060.
Khan, S., Zou, Y., Amjad, A., Gardezi, A., Smith, C. L., Winters, C., & Reese, T. S. (2011). Sequestration of CaMKII in dendritic spines in silico. Journal of Computational Neuroscience, 31, 581–594.
Kolodziej, S. J., Hudmon, A., Waxham, M. N., & Stoops, J. K. (2000). Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIalpha and truncated CaM kinase IIalpha reveal a unique organization for its structural core and functional domains. Journal of Biological Chemistry, 275, 14354–14359.
Lee, S. J., Escobedo-Lozoya, Y., Szatmari, E. M., & Yasuda, R. (2009). Activation of CaMKII in single dendritic spines during long-term potentiation. Nature, 458, 299–304.
Lippincott-Schwartz, J., Altan-Bonnet, N., Patterson, G.H., (2003). Photobleaching and photoactivation: following protein dynamics in living cells. Nature Cell Biology Suppl, S7–14.
Lisman, J. E., Raghavachari, S., & Tsien, R. W. (2007). The sequence of events that underlie quantal transmission at central glutamatergic synapses. Nature Reviews. Neuroscience, 8, 597–609.
Noguchi, J., Matsuzaki, M., Ellis-Davies, G. C., & Kasai, H. (2005). Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron, 46, 609–622.
Northrup, S. H., & Erickson, H. P. (1992). Kinetics of protein-protein association explained by Brownian dynamics computer simulation. Proceedings of the National Academy of Sciences of the United States of America, 89, 3338–3342.
Okabe, S. (2007). Molecular anatomy of the postsynaptic density. Molecular and Cellular Neuroscience, 34, 503–518.
Okamoto, K., Narayanan, R., Lee, S. H., Murata, K., & Hayashi, Y. (2007). The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proceedings of the National Academy of Sciences of the United States of America, 104, 6418–6423.
Okamoto, K., Bosch, M., & Hayashi, Y. (2009). The roles of CaMKII and F-actin in the structural plasticity of dendritic spines: a potential molecular identity of a synaptic tag? Physiology (Bethesda, Md.), 24, 357–366.
O’Leary, H., Lasda, E., & Bayer, K. U. (2006). CaMKIIbeta association with the actin cytoskeleton is regulated by alternative splicing. Molecular Biology of the Cell, 17, 4656–4665.
Otmakhov, N., Tao-Cheng, J. H., Carpenter, S., Asrican, B., Dosemeci, A., Reese, T. S., & Lisman, J. (2004). Persistent accumulation of calcium/calmodulin-dependent protein kinase II in dendritic spines after induction of NMDA receptor-dependent chemical long-term potentiation. Journal of Neuroscience, 24, 9324–9331.
Ouyang, Y., Wong, M., Capani, F., Rensing, N., Lee, C. S., Liu, Q., Neusch, C., Martone, M. E., Wu, J. Y., Yamada, K., Ellisman, M. H., & Choi, D. W. (2005). Transient decrease in F-actin may be necessary for translocation of proteins into dendritic spines. The European Journal of Neuroscience, 22, 2995–3005.
Patterson, G. H., & Lippincott-Schwartz, J. (2002). A photoactivatable GFP for selective photolabeling of proteins and cells. Science, 297, 1873–1877.
Pi, H. J., Otmakhov, N., El Gaamouch, F., Lemelin, D., De Koninck, P., & Lisman, J. (2010). CaMKII control of spine size and synaptic strength: role of phosphorylation states and nonenzymatic action. Proceedings of the National Academy of Sciences of the United States of America, 107, 14437–14442.
Rajpoot, N. M., Wilson, R. G., Meyer, F. G., & Coifman, R. R. (2003). Adaptive wavelet packet basis selection for zerotree image coding. IEEE Transactions on Image Processing, 12, 1460–1472.
Rajpoot, N. M., Yao, Z., & Wilson, R. G. (2004). Adaptive wavelet restoration of noisy video sequenes. Proceedings of the International Conference Image Processing, 2, 957–960.
Sanabria, H., Digman, M. A., Gratton, E., & Waxham, M. N. (2008). Spatial diffusivity and availability of intracellular calmodulin. Biophysical Journal, 95, 6002–6015.
Sanabria, H., Swulius, M. T., Kolodziej, S. J., Liu, J., & Waxham, M. N. (2009). {beta}CaMKII regulates actin assembly and structure. Journal of Biological Chemistry, 284, 9770–9780.
Schaus, T. E., Taylor, E. W., & Borisy, G. G. (2007). Self-organization of actin filament orientation in the dendritic-nucleation/array-treadmilling model. Proceedings of the National Academy of Sciences of the United States of America, 104, 7086–7091.
Sharma, K., Fong, D. K., & Craig, A. M. (2006). Postsynaptic protein mobility in dendritic spines: long-term regulation by synaptic NMDA receptor activation. Molecular and Cellular Neuroscience, 31, 702–712.
Shen, K., & Meyer, T. (1998). In vivo and in vitro characterization of the sequence requirement for oligomer formation of Ca2+/calmodulin-dependent protein kinase IIalpha. Journal of Neurochemistry, 70, 96–104.
Shen, K., & Meyer, T. (1999). Dynamic control of CaMKII translocation and localization in hippocampal neurons by NMDA receptor stimulation. Science, 284, 162–166.
Shen, K., Teruel, M. N., Subramanian, K., & Meyer, T. (1998). CaMKIIbeta functions as an F-actin targeting module that localizes CaMKIIalpha/beta heterooligomers to dendritic spines. Neuron, 21, 593–606.
Shen, K., Teruel, M. N., Connor, J. H., Shenolikar, S., & Meyer, T. (2000). Molecular memory by reversible translocation of calcium/calmodulin-dependent protein kinase II. Nature Neuroscience, 3, 881–886.
Sheng, M., & Hoogenraad, C. C. (2007). The postsynaptic architecture of excitatory synapses: a more quantitative view. Annual Review of Biochemistry, 76, 823–847.
Shroff, H., Galbraith, C. G., Galbraith, J. A., & Betzig, E. (2008). Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nature Methods, 5, 417–423.
Yang, L., Parton, R., Ball, G., Qiu, Z., Greenaway, A. H., Davis, I., & Lu, W. (2010). An adaptive non-local means filter for denoising live-cell images and improving particle detection. Journal of Structural Biology, 172, 233–243.
Acknowledgments
We thank Dr Steven Andrews for advice and discussion regarding Smoldyn and Dr Ayse Dosemici for comments on the manuscript. Ayisha Shabbir was supported by start-up funds from the LUMS School of Science & Engineering (to S.K).
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Upinder Singh Bhalla
Supplemental materials
Below is the link to the electronic supplementary material.
(PAalpha.mpg): Movie of de-noised image sequence showing sequestration of GFP*-CaMKIIa to spine tip (MPG 5488 kb)
(PAbetaK.mpg): Movie of de-noised image sequence showing sequestration of GFP*-CaMKIIb to spine body. (MPG 3786 kb)
(PAgfp.mpg): Movie of Smoldyn simulation demonstrating trapping of GFP* in mushroom spine. (MPG 4474 kb)
Script 1
(spine-PAgfpS1D80.txt): Example Smoldyn script for computation of GFP*-CaMKIIa residence times. (TXT 3 kb)
Script 2
(spine-PAgfpS2.txt): Example Smoldyn script for computation of GFP*-CaMKIIb residence times. Surface and solution reactions with the dendritic cortical actin and spine actin cytoskeleton are included to the reactions in Script 1. The dendrite geometry is different in the two scripts to illustrate virtual cell set-up (PDF 44.3 kb)
Rights and permissions
About this article
Cite this article
Khan, S., Reese, T.S., Rajpoot, N. et al. Spatiotemporal maps of CaMKII in dendritic spines. J Comput Neurosci 33, 123–139 (2012). https://doi.org/10.1007/s10827-011-0377-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-011-0377-1