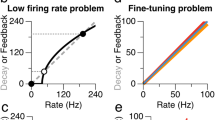
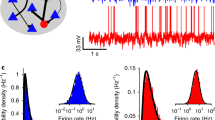
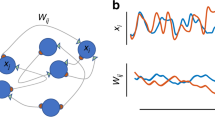
Abstract
Recurring sequences of neuronal activation in the hippocampus are a candidate for a neurophysiological correlate of episodic memory. Here, we discuss a mean-field theory for such spike sequences in phase space and show how they become unstable when the neuronal network operates at maximum memory capacity. We find that inhibitory feedback rescues replay of the sequences, giving rise to oscillations and thereby enhancing the network’s capacity. We further argue that transient sequences in an overloaded network with feedback inhibition may provide a mechanistic picture of memory-related neuronal activity during hippocampal sharp-wave ripple complexes.






Similar content being viewed by others
References
Amit, Y., & Huang, Y. (2010). Precise capacity analysis in binary networks with multiple coding level inputs. Neural Computation, 22, 660–688.
Buhry, L., Azizi, A. H., & Cheng, S. (2011). Reactivation, replay, and preplay: How it might all fit together. Neural Plasticity, 2011, 203462.
Buzsáki, G., Horvath, Z., Urioste, R., Hetke, J., & Wise, K. (1992). High-frequency network oscillation in the hippocampus. Science, 256, 1025–1027.
Colgin, L. L. (2011). Oscillations and hippocampal-prefrontal synchrony. Current Opinion in Neurobiology, 21, 467–474.
Csicsvari, J., Hirase, H., Mamiya, A., & Buzsáki, G. (2000) Ensemble patterns of hippocampal CA3-CA1 neurons during sharp wave-associated population events. Neuron, 28, 585–594.
Davidson, T. J., Kloosterman, F., & Wilson, M. A. (2009). Hippocampal replay of extended experience. Neuron, 63, 497–507.
Dragoi, G., & Tonegawa, S. (2011). Preplay of future place cell sequences by hippocampal cellular assemblies. Nature, 469, 397–401.
Diba, K., & Buzsáki, G (2007). Forward and reverse hippocampal place-cell sequences during ripples. Nature Neuroscience, 10, 1241–1242.
Dragoi, G., & Buzsáki, G. (2006). Temporal encoding of place sequences by hippocampal cell assemblies. Neuron, 50, 145–157
Gardner, E. (1987). Maximum storage capacity in neural networks. Europhysics letters, 4, 481–485.
Gibson, W. G., & Robinson, J. (1992). Statistical analysis of the dynamics of a sparse associative memory. Neural Networks, 5, 645–661.
Golomb, D., Rubin, N., & Sompolinsky, H. (1990). Willshaw model: Associative memory with sparse coding and low firing rates. Physical Review. A, 41, 1843–1854.
Gupta, A. S., van der Meer, M. A., Touretzky, D. S., & Redish, A. D. (2010). Hippocampal replay is not a simple function of experience. Neuron, 65, 695–705.
Hirase, H., & Recce, M. (1996). A search for the optimal thresholding sequence in an associative memory. Network, 7, 741–756.
Jutras, M. J., & Buffalo, E. A. (2010). Synchronous neural activity and memory formation. Current Opinion in Neurobiology, 20, 150–155.
Latham, P. E., & Nirenberg, S. (2004). Computing and stability in cortical networks. Neural Computation, 16, 1385–1412.
Lee, A. K., & Wilson, M. A. (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron, 36, 1183–1194.
Leibold, C., & Kempter, R. (2006) Memory capacity for sequences in a recurrent network with biological constraints. Neural Computation, 18, 904–941.
Maier, N., Tejero-Cantero, Á., Dorrn, A., Winterer, J., Beed, P., Morris, G., et al. (2011). Coherent phasic excitation during hippocampal ripples. Neuron, 72, 137–152.
Memmesheimer, R. (2010). Quantitative prediction of intermittent high-frequency oscillations in neural networks with supralinear dendritic interactions. Proceedings of the National Academy of Sciences of the United States of America, 107, 11092–11097.
Miles, R., & Wong, R. K. (1986). Excitatory synaptic interactions between CA3 neurones in the guinea-pig hippocampus. Journal of Physiology, 373, 397–418.
Nadal, J.-P. (1991). Associative memory: On the (puzzling) sparse coding limit. Journal of Physics. A. Mathematical and General, 24, 1093–1101.
Nádasdy, Z., Hirase, H., Czurkó, A., Csicsvari, J., & Buzsáki, G. (1999). Replay and time compression of recurring spike sequences in the hippocampus. Journal of Neuroscience, 21, 9497–9507.
O’Keefe, J., & Dostrovsky, J. (1971). The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Research, 34, 171–175.
Schmitz, D., Schuchmann, S., Fisahn, A., Draguhn, A., Buhl, E., Petrasch-Parwez, E., et al. (2001). Axo-axonal coupling: A novel mechanism for ultrafast neuronal communication. Neuron, 31, 831–840.
Sigurdsson, T., Stark, K. L., Karayiorgou, M., Gogos, J. A., & Gordon, J. A. (2010). Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature, 464, 763–767.
Sullivan, S., Csicsvari, J., Mizuseki, K., Montgomery, S., Diba, K., & Buzsáki, G. (2011). Relationships between hippocampal sharp waves, ripples, and fast gamma oscillation: Influence of dentate and entorhinal cortical activity. Journal of Neuroscience, 31, 8605–8616.
Vladimirov, N., Tu, Y., & Traub, R. D. (2012). Shortest loops are pacemakers in random networks of electrically coupled axons. Frontiers in Computational Neuroscience, 6, 17.
Treves, A. (1990). Graded-response neurons and information encodings in autoassociative memories. Physical Review A, 42, 2418–2430.
Tsodyks, M. V., & Feigel’man, M. V. (1988). Enhanced storage capacity in neural networks with low level of activity. Europhysics Letters, 6, 101–105.
Wang, X.-J. (2010). Neurophysiological and computational principles of cortical rhythms in cognition. Physiological Reviews, 90, 1195–1268.
Willshaw, D. J., Buneman, O. P., & Longuet-Higgins, H. C. (1969). Non-holographic associative memory. Nature, 222, 960–962.
Wilson, M. A., & McNaughton, B. L. (1994) Reactivation of hippocampal ensemble memories during sleep. Science, 265, 676–679.
Acknowledgements
The authors are grateful to Alexander Mathis for comments on a previous version of the manuscript. This work was funded by the German Ministry for Education and Research (BMBF) under grant number 01GQ0440 (BCCN Munich) and the German Science Foundation (DFG) under grant numbers Le-2250/2 and GRK 1190.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: David Golomb
Axel Kammerer and Álvaro Tejero-Cantero contributed equally to this paper.
Appendix: First and second moments
Appendix: First and second moments
The dynamics underlying neuronal activity sequences is formulated as a two-dimensional iterated map in Eqs. (2)–(6). This time-discrete dynamics is simplified using Gaussian approximations for the distributions of the number h of synaptic inputs to a specified neuron. The Gaussian approximation therefore requires expressions for the means and variances of the input sums h.
Inputs can be of two kinds, hits m and false alarms n. The input sum \(h=\sum_{j=1}^{m+n}w_{j}\, s_{j}\) thus runs over all m + n ≤ N active (firing) neurons in the network and depends on two binary random variables for each potential input: w ∈ {0,1} indicating the presence of a synaptic connection, and s ∈ {0,1} indicating its state (Gibson and Robinson 1992). The stochasticity of s is inherited from the randomness of the activity patterns underlying the memory sequences via Willshaw’s learning rule.
The distribution of w is given by the morphological connectivity such that prob(w = 1) = c m . The probability prob(s = 1) of a synapse having been potentiated depends on whether it connects or not neurons that should fire in sequence at the particular point in time.
The Willshaw rule ensures that synapses that connect sequentially firing neurons are in the potentiated state, i.e. prob(s = 1) = 1, and thus for this subset of synapses the input sum depends on a binomial process with probability prob(w = 1) = c m .
For the other synapses, the probability prob(s = 1) = q x depends on the the number x ≤ P of associations the specific postsynaptic neuron is involved in. Note that if the postsynaptic neuron is never supposed to fire, the Willshaw rule will activate none of its synapses and thus q 0 = 0. In general, the probability that a neuron is not a target in one specific step of the sequence (association) is 1 − f, and thus the probability that it is not a target in any one of x associations is (1 − f)x. Conversely, the probability of such a synapse being potentiated is \(q_{x}=1-(1-f)^{x}\). Hence, assuming independence of the two binomial processes, the input sum h for this subset of synapses is binomial with probability
The probability distribution of the input h can then be determined as
in which the conditional probability \(p(h|x)=\binom{m+n}{h}\,(c_{m}\ q_{x})^{h}\,(1-c_{m} q_{x})^{m+n-h}\) is derived from Eq. (11), and the probability p(x) that a neuron is involved in x associations is also binomial, viz. \(p(x)=\binom{P}{x}\, f^{x}\,(1-f)^{P-x}\).
To compute expected values of h, we have to discern between neurons that should be active at time step t + 1 (and are supposed to generate the hits) and those that should be silent (and potentially give rise to false alarms). For the potential false alarms, we obtain
Note that the last step makes use of the capacity of the Willshaw rule, Eq. (1). Similarly, for the potential hits, we obtain
Here the expected value sums over two independent subsets of neurons, the first one (h′) representing the false alarms, and the second (h′′) representing the hits during the previous time step.
The corresponding variances can be obtained analogously employing the formula of the geometric series several times, and introducing the abbreviation \({\rm CV}^2_{q}={\rm var}_{x}\ q/\langle q\rangle_{x}^{2}\) with expected values according to the distribution p(x):
Note that CV q →0 for f→0, and, in this limit, the variance formulas \(\sigma_{\rm On}^{2}\to m\, c_{m}\,(1-c_{m})+n\, c\,(1-c)\), \(\sigma_{\rm Of\/f}^{2}\to(m+n)\, c\,(1-c)\) from the present theory approximate those in Leibold and Kempter (2006).
Rights and permissions
About this article
Cite this article
Kammerer, A., Tejero-Cantero, Á. & Leibold, C. Inhibition enhances memory capacity: optimal feedback, transient replay and oscillations. J Comput Neurosci 34, 125–136 (2013). https://doi.org/10.1007/s10827-012-0410-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-012-0410-z