Abstract
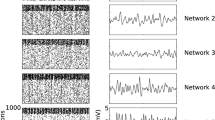
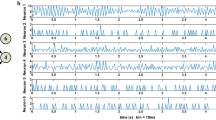
Granger causality (GC) analysis has emerged as a powerful analytical method for estimating the causal relationship among various types of neural activity data. However, two problems remain not very clear and further researches are needed: (1) The GC measure is designed to be nonnegative in its original form, lacking of the trait for differentiating the effects of excitations and inhibitions between neurons. (2) How is the estimated causality related to the underlying synaptic weights? Based on the GC, we propose a computational algorithm under a best linear predictor assumption for analyzing neuronal networks by estimating the synaptic weights among them. Under this assumption, the GC analysis can be extended to measure both excitatory and inhibitory effects between neurons. The method was examined by three sorts of simulated networks: those with linear, almost linear, and nonlinear network structures. The method was also illustrated to analyze real spike train data from the anterior cingulate cortex (ACC) and the striatum (STR). The results showed, under the quinpirole administration, the significant existence of excitatory effects inside the ACC, excitatory effects from the ACC to the STR, and inhibitory effects inside the STR.










Similar content being viewed by others
Notes
The i − th equation of the reduced model reads \({x_{t}^{i}}=\sum \limits _{r=1}^{p}b_{r}^{i,1}x_{t-r}^{1}+\cdots +\sum \limits _{r=1}^{p}b_{r}^{i,j-1}x_{t-r}^{j-1}+ \sum \limits _{r=1}^{p}b_{r}^{i,j+1}x_{t-r}^{j+1}+\cdots +\sum \limits _{r=1}^{p}b_{r}^{i,n}x_{t-r}^{n}+ \eta ^{\textbf {i,j}}\), where the b’s are the corresponding projection coefficients.
This means that the estimated weight vector \(\hat \alpha ^{i}=(\hat \alpha _{i_{1}},\hat \alpha _{i_{2}},\cdots ,\hat \alpha _{i_{k}})\) is normalized by its l 1 norm \(\|\hat \alpha ^{i}\|_{1}:=|\hat \alpha _{i_{1}}|+|\hat \alpha _{i_{2}}|+\cdots + |\hat \alpha _{i_{k}}|\). Then the normalized weight vector \(\hat \alpha ^{i}/\|\hat \alpha ^{i}\|_{1}\) will have unit l 1 vector norm.
References
Arnold, A., Liu, Y., & Abe, N. (2007). Temporal causal modeling with graphical Granger methods. Proceedings of the 13th ACM SIGKDD international conference on Knowledge discovery and data mining, 7, 66–75.
Baccala, L.A., & Sameshima, K. (2001). Partial directed coherence : a new concept in neural stucture determination. Biological Cybernetics, 84, 463–474.
Barnett, L., & Seth, A.K. (2014). The MVGC multivariate Granger causality toolbox: A new approach to Granger-causal inference. Journal of Neuroscience Methods, 223, 50–68.
Barrett, A.B., & Barnett, L. (2013). Granger causality is designed to measure effect, not mechanism. Frontiers in Neuroinformatics, 7, 1–2.
Benjamini, Y., & Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, 57, 289–300.
Bressler, S.L., & Seth, A.K. (2011). Wiener-Granger Causality: A well established methodology. NeuroImage, 58, 323–329.
Cadotte, A.J., DeMarse, T.B., He, P., & Ding, M. (2008). Causal measures of structure and plasticity in simulated and living neural networks. PLoS Computational Biology, 3, 1–14.
Cadotte, A.J., DeMarse, T.B., Mareci, T.H., Parekh, M.B., Talathi, S.S., Hwang, D.U., Ditto, W.L., Ding, M., & Carney, P.R. (2010). Granger causality relationships between local field potentials in an animal model of temporal lobe epilepsy. Journal of Neuroscience Methods, 189, 121–129.
Dhamala, M., Rangarajan, G., & Ding, M. (2008). Analyzing information flow in brain networks with nonparametric Granger causality. NeuroImage, 41, 354–362.
Ding, M., Chen, Y., & Bressler, S.L. (2006). Granger Causality: Basic Theory and Application to Neuroscience. Handbook of Time Series Analysis: Recent Theoretical Developments and Applications, (pp. 437–460). Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA.
Gomez, L., Budelli, R., Saa, R., Stiber, M., & Segundo, J.P. (2005). Pooled spike trains of correlated presynaptic inputs as realizations of cluster point processes. Biological Cybernetics, 92, 110–127.
Granger, C. (1969). Investigating causal relations by econometric models and cross-spectral methods. Econometrica, 37, 424–438.
Granger, C. (1980). Testing for causality: A personal viewpoint. Journal of Economic Dynamics and Control, 2, 329–352.
Greene, W.H. (2002). Econometric Analysis, fifth ed. Upper Saddle River, NJ: Prentice-Hall.
Guo, S., Seth, A.K., Kendrick, K.M., Zhou, C., & Feng, J. (2008). Partial Granger causality – Eliminating exogenous inputs and latent variables. Journal of Neuroscience Methods, 172, 79–93.
Hu, S., Dai, G., Worrel, G. A., Dai, Q., & Liang, H. (2011). Causality analysis of neural connectivity: critical examination of existing methods and advances of new methods. IEEE Transactions on Neural Networks, 22, 829–844.
Huang, J.J., Yen, C.T., Liu, T.L., Tsao, H.W., Hsu, J.W., & Tsai, M.L. (2013). Effects of dopamine D2 agonist quinpirole on neuronal activity of anterior cingulate cortex and striatum in rats. Psychopharmacology, 227, 459–466.
Izhikevich, E.M. (2003). Simple models of spiking neurons. IEEE Transactions on Neural Networks, 14, 1569–1572.
Kim, S., Putrino, D., Ghosh, S., & Brown, E.N. (2011). A Granger causality measure for point process models of ensemble neural spiking activity. PLoS Computational Biology, 7, 3.
Kitagawa, G. (2010). Introduction to Time Series Modeling: Chapman & Hall/CRC Monographs on Statistics & Applied Probability.
Krumin, M., & Shoham, S. (2010). Multivariate autoregressive modeling and Granger causality analysis of multiple spike trains. Computational Intelligence and Neuroscience, 752428.
Lehky, S.R. (2010). Decoding poisson spike trains by gaussian filtering. Neural Computation, 22, 1245–1271.
Luo, Q., Lu, W., Cheng, W., Valdes-Sosa, P.A., Wen, X., Ding, M., & Feng, J. (2013). Spatio-temporal Granger causality: A new framework. NeuroImage, 79, 241–263.
Lutkepohl, H. (2005). New Introduction to Multiple Time Series Analysis: Springer.
Marinazzo, D., Liao, W., Chen, H., & Stramaglia, S. (2011). Nonlinear connectivity by Granger causality. NeuroImage, 58, 330–338.
Michailidis, G., & d’Alche-Buc, F. (2013). Autoregressive models for gene regulatory network inference: Sparsity, stability and causality issues. Mathematical Biosciences, 246, 326–334.
Nageswaran, J.M., Dutt, N., Krichmar, J.L., Nicolau, A., & Veidenbaum, A.V. (2009). A configurable simulation environment for the efficient simulation of large-scale spiking neural networks on graphics processors. Neural Networks, 22, 791–800.
Nedungadi, A.G., Rangarajan, G., Jain, N., & Ding, M. (2009). Analyzing multiple spike trains with nonparametric granger causality. Journal of Computational Neuroscience, 27, 55–64.
Pillow, J.W., Shlens, J., Paninski, L., Sher, A., Litke, A.M, Chichilnisky, E.J., & Simoncelli, E.P. (2008). Spatio-temporal correlations and visual signalling in a complete neuronal population. Nature, 454, 995–999.
Quinn, C.J., Coleman, T.P., Kiyavash, N., & Hatsopoulos, N.G. (2011). Estimating the directed information to infer causal relationships in ensemble neural spike train recordings. Journal of Computational Neuroscience, 30, 17–44.
Rosenbaum, R., Trousdale, J., & Josic, K. (2011). The effects of pooling on spike train correlations. Frontiers in Neuroscience, 5, 1–10.
Sameshima, K., & Baccala, L. A. (1999). Using partial directed coherence to describe neuronal ensemble interactions. Journal of Neuroscience Methods, 94, 93–103.
Seth, A.K. (2005). Causal connectivity of evolved neural networks during behavior. Network, 16, 35–54.
Seth, A.K., & Edelman, G.M. (2007). Distinguishing causal interactions in neural populations. Neural Computation, 19, 910–933.
Seth, A.K. (2010). A MATLAB toolbox for Granger causal connectivity analysis. Journal of Neuroscience Methods, 186, 262–273.
Shao, P.C., Tseng, W.T., Kuo, C.C., Shann, W.C., Tsai, M.L., & Yen, C.C. (2013). Effects of spike sorting error on the Granger causality index. Neural Networks, 46, 249–259.
Shimazaki, H., & Shinomoto, S. (2007). A method for selecting the bin size of a time histogram. Neural Computation, 19, 1503– 1527.
Zhou, D., Xiao, Y., Zhang, Y., Xu, Z., & Cai, D. (2014). Granger causality network reconstruction of conductance-based integrate-and-fire neuronal systems. PLoS ONE, 9, 2.
Zhu, L., Lai, Y.C., Hoppensteadt, F.C., & He, J. (2003). Probing changes in neural interaction during adaptation. Neural Computation, 15, 2359–2377.
Zou, C., Ladroue, C., Guo, S., & Feng, J. (2010). Identifying interactions in the time and frequency domains in local and global networks - A Granger causality approach. BMC Bioinformatics, 11, 337.
Acknowledgments
The authors would like to thank the anonymous reviewers and editors for their valuable comments, which led to a clearer presentation. This work was supported by the National Science Council of Taiwan under the grants NSC-101-2115-M-030-004, NSC-102-2313-B-197-001, NSC-102-2633-B-029-001, MOST 103-2633-B-029-001, and the Fu Jen Catholic University under the grant A0502004. The Matlab code used for this study is available to interested readers upon request.
Conflict of interests
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Action Editor: Rob Kass
Appendix: Derivation of the NSI using simple network
Appendix: Derivation of the NSI using simple network
Here, we re-formulate the NSI using the simple network (Fig. 1). Let u = α x + β y + γ z form the BLP of w, then there exist p, {f r , r = 1, 2, ⋯, p}, and {d r , r = 1, 2, ⋯, p} such that \(w_{t}={\sum }_{r=1}^{p}[f_{r}u_{t-r}+d_{r}w_{t-r}]+\epsilon _{t}\), where 𝜖 is a stationary white noise possessing the smallest variance among \(\mathcal {G} =span(\{x,y,z,v_{1},v_{2},v_{3}\})\). Replacing u with the weighted trajectory, we obtain
On the other hand, fitting to data the following empirical regression
where \(g_{r}v_{t-r}:={\sum }_{k=1}^{3}g_{k,r}v_{k,t-r}\) for convenience.
-
If v is stochastically independent of x,y,z,w, then we have g r ≡ 0. Since {a r },{b r },{c r } can be obtained through Least-Squares method, comparing Eq. (A.2) with Eq. (A.1), we have
$$ \sum\limits_{r=1}^{p}a_{r}=\alpha\sum\limits_{r=1}^{p} f_{r},\> \sum\limits_{r=1}^{p}b_{r}=\beta\sum\limits_{r=1}^{p} f_{r},\> \sum\limits_{r=1}^{p}c_{r}=\gamma\sum\limits_{r=1}^{p} f_{r}, $$(A.3)and get
$$ \alpha:\beta:\gamma=\sum\limits_{r=1}^{p}a_{r}:\sum\limits_{r=1}^{p}b_{r}:\sum\limits_{r=1}^{p}c_{r},\> \text{provided}\> \sum\limits_{r=1}^{p}f_{r}> 0, $$(A.4)where \(sgn(\alpha )=sgn\left (\sum \limits _{r=1}^{p}a_{r}\right )\), \(sgn(\beta )=sgn\left (\sum \limits _{r=1}^{p}b_{r}\right )\), and \(sgn(\gamma )=sgn\left (\sum \limits _{r=1}^{p}c_{r}\right )\).
-
If v is linear dependent of x,y,z,w, then g r ≫0 and {a r },{b r },{c r } will be affected. However, since 𝜖 in Eq. (A.1) possessing the smallest variance among \(\mathcal {G}\), taking out v does not increase the variance of \(\tilde {\epsilon }\), therefore we still can correct the model coefficients by ruling out the useless information v.
Finally, the neuron synaptic index from x, y, z to w are defined respectively as
where |N x → w |+|N y → w |+|N z → w | = F u → w is the GC index from the weighted trajectory u = α x + β y + γ z to the target trajectory w.
Rights and permissions
About this article
Cite this article
Shao, PC., Huang, JJ., Shann, WC. et al. Granger causality-based synaptic weights estimation for analyzing neuronal networks. J Comput Neurosci 38, 483–497 (2015). https://doi.org/10.1007/s10827-015-0550-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-015-0550-z