Abstract
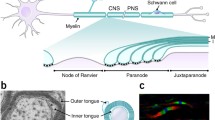
Multilayered, lipid-rich myelin increases nerve impulse conduction velocity, contributes to compact nervous systems, and reduces metabolic costs of neural activity. Based on the hypothesis that increased impulse conduction velocity provides a selective advantage that drives the evolution of myelin, we simulated a sequence of plausible intermediate stages of myelin evolution, each of which providing an enhancement of conduction speed. We started with the expansion of insulating glial coverage, which led first to a single layer of myelin surrounding the axon and then to multiple myelin wraps with well-organized nodes. The myelinated fiber was modeled at three levels of complexity as the hypothesized evolutionary progression became more quantitatively exacting: 1) representing the fiber as a mathematically-tractable uniform active cylinder with the effect of myelination approximated by changing its specific capacitance (C m ); 2) representing it as a chain of simple, cable-model compartments having alternating nodal and internodal parameters subject to optimization, and 3) representing it in a double cable model with the axon and myelin sheath treated separately. Conduction velocity was optimized at each stage. To maintain optimal conduction velocities, increased myelin coverage of axonal surface must be accompanied by an increase in channel density at the evolving nodes, but along with increases in myelin thickness, a reduction in overall average channel density must occur. Leakage under the myelin sheath becomes more of a problem with smaller fiber diameters, which may help explain the tendency for myelin to occur preferentially in larger nerve fibers in both vertebrates and invertebrates.










Similar content being viewed by others
References
Bakiri, Y., Káradóttir, R., Cossell, L., & Attwell, D. (2011). Morphological and electrical properties of oligodendrocytes in the white matter of the corpus callosum and cerebellum. Journal of Physiology, 589, 559–573.
Binstock, L., Adelman, W. J., Senft, J. P., & Lecar, H. (1975). Determination of the resistance in series with the membrane of giant axons. Journal of Membrane Biology, 21, 25–47.
Blight, A. R. (1985). Computer simulation of action potentials and afterpotentials in mammalian myelinated axons: the case for a lower resistance myelin sheath. Neuroscience, 15, 13–31.
Brill, M. H., Waxman, S. D., Moore, J. W., & Joyner, R. W. (1977). Conduction velocity and spike configuration in myelinated fibres: computed dependence on internode distance. Journal of Neurology, Neurosurgery and Psychiatry, 40, 769–774.
Brösamle, C., & Halpern, M. E. (2002). Characterization of myelination in developing zebrafish. Glia, 39, 47–57.
Bullock, T. H., & Horridge, G. A. (1965). Structure and Function in the Nervous System of Invertebrates (Vol. 1). San Francisco: W. H. Freeman.
Carnevale, N. T., & Hines, M. L. (2005). The NEURON Book. Cambridge, U.K.: Cambridge University Press.
Carpenter, F. G., & Bergland, R. M. (1975). Excitation and conduction in immature nerve fibers of the developing chick. American Journal of Physiology, 190, 371–376.
Chomiak, T., & Hu, B. (2009). What is the optimal value of the g-ratio for myelinated fibers in the rat CNS? A theoretical approach. PLoS One, 4, e7754.
Crotty, P., Sangrey, T. D., & Levy, W. B. (2006). Metabolic energy cost of action potential velocity. Journal of Neurophysiology, 96, 1237–1246.
Davis, A. D., Weatherby, T. M., Hartline, D. K., & Lenz, P. H. (1999). Myelin-like sheaths in copepod axons. Nature, 398, 571.
Devaux, J., & Gow, A. (2008). Tight junctions potentiate the insulative properties of the small CNS myelinated axons. The Journal of Cell Biology, 183, 909–921.
Eaton, R. C. (Ed.). (1984). Neural Mechanisms of Startle Behavior. New York, NY: Plenum Press.
Fields, R. D. (2008). White matter in learning, cognition and psychaitric disorders. Trends in Neurosciences, 31, 361–370.
Fitzhugh, R. (1962). Computation of impulse initiation and saltatory conduction in a myelinated nerve fiber. Biophysical Journal, 2, 11–21.
Frankenhaeuser, B., & Huxley, A. F. (1964). The action potential in the myelinated nerve fibre of Xenopus laevis as computed on the basis of voltage clamp data. Journal of Physiology, 171, 302–315.
Freidländer, B. (1889). Über die markhaltigen Nervenfasern und Neurochorde der Crustaceen und Anneliden. Mittheilungen aus der Zoologischen Station zu Neapel, 9, 205–265.
Gasser, H. S., & Grundfest, H. (1939). Axon diameters in relation to the spike dimensions and the conduction velocity in mammalian A fibers. American Journal of Physiology, 127, 393–414.
Goldman, L., & Albus, J. S. (1968). Computation of impulse conduction in myelinated fibers: Theoretical basis of the velocity-diameter relation. Biophysical Journal, 8, 596–607.
Günther, J. (1976). Impulse conduction in the myelinated giant fibers of the earthworm. Structure and function of the dorsal nodes in the median giant fiber. Journal of Comparative Neurology, 168, 505–532.
Halter, J. A., & Clark, J. W. (1991). A distributed-parameter model of the myelinated nerve fiber. Journal of Theoretical Biology, 148, 345–382.
Halter, J. A., & Clark, J. W. (1993). The influence of nodal constriction on conduction velocity in myelinated nerve fibers. NeuroReport, 4, 89–92.
Hama, K. (1959). Some observations on the fine structure of the giant fibers of the earth worm Eisenia foetida. Journal of Biophysical and Biochemical Cytology 6, 61–66.
Harris, J. J., & Attwell, D. (2012). The energetics of CNS white matter. Journal of Neuroscience, 32, 356–371.
Hartline, D. K. (2008). What is myelin? Neuron Glia Biology, 4, 153–163.
Hartline, D. K., & Colman, D. K. (2007). Rapid conduction and the evolution of giant axons and myelinated fibers. Current Biology, 17, R29–R35.
Hartline, D. K., & Kong, J. H. (2008). The gain and loss of myelin in malacostracan crustaceans. In Program No. 79.22. 2008 Neuroscience Meeting Planner. Washington, DC: Society for Neuroscience.
Hasenstaub, A., Otte, S., Callaway, E., & Sejnowski, T. J. (2010). Metabolic cost as a unifying principle governing neuronal biophysics. Proceedings of the National Academy of Science, 107, 12329–12334.
Hines, M. L., & Carnevale, N. T. (1997). The NEURON simulation environment. Neural Computation, 9, 1179–1209.
Hines, M. L., & Shrager, P. (1991). A computational test of the requirements for conduction in demyelinated axons. Restorative Neurology and Neuroscience, 3, 81–93.
Hodgkin, A. L. (1947). The membrane resistance of a non-medullated nerve fibre. Journal of Physiology, 106, 305–318.
Hodgkin, A. L. (1975). The optimum density of sodium channels in an unmyelinated axon. Philosophical Transactions of the Royal Society B, 270, 297–300.
Hodgkin, A. L., & Huxley, A. F. (1952). A quantitative description of membrane current and its application to conduction and excitation in nerve. Journal of Physiology, 117, 500–544.
Huxley, A. F. (1959). Ion movements during nerve activity. Annals of the New York Academy of Sciences, 81, 221–246.
Huxley, A. F., & Stämpfli, R. (1949). Evidence for saltatory conduction in peripheral myelinated nerve fibres. Journal of Physiology, 108, 315–339.
Keynes, R. D., & Rojas, E. (1974). Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. Journal of Physiology, 239, 393–434.
Koles, Z. J., & Rasminsky, M. (1972). A computer simulation of conduction in demyelinated fibres. Journal of Physiology, 227, 351–364.
Lasiene, J., Shupe, L., Perlmutter, S., & Horner, P. (2008). No evidence for chronic demyelination in spared axons after spinal cord injury in a mouse. Journal of Neuroscience, 28, 3887–3896.
Lasiene, J., Matsui, A., Sawa, Y., Wong, F., & Horner, P. J. (2009). Age-related myelin dynamics revealed by increased oligodendrogenesis and short internodes. Aging Cell, 8, 201–213.
McIntyre, C. C., Richardson, A. G., & Grill, W. M. (2002). Modeling the excitability of mammalian nerve fibers: Influence of afterpotentials on the recovery cycle. Journal of Neurophysiology, 87, 995–1006.
Moore, J. W., Joyner, R. W., Brill, M. H., Waxman, S. D., & Najar-Joa, M. (1978). Simulations of conduction in uniform myelinated fibers: Relative sensitivity to changes in nodal and internodal parameters. Biophysical Journal, 21, 147–160.
Nageotte, J. (1916). Notes sur les fibres à myeline et sur les étranglements de Ranvier chez certains crustacés. Comptes Rendus des Seances de la Societe de Biologie et de Ses Filiales, 79, 259–263.
Novakovic, S. D., Levinson, S. R., Schachner, M., & Shrager, P. (1998). Disruption and reorganization of sodium channels in experimental allergic neuritis. Muscle & Nerve, 21, 1019–1032.
Pajevic, S., Basser, P. J., & Fields, R. D. (2014). Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience, 276, 135–147.
Peters, A., & Vaughn, J. E. (1970). Morphology and development of the myelin sheath. In A. N. Davison & A. Peters (Eds.), Myelination (pp. 3–79). Springfield, IL: Charles C. Thomas.
Poliak, S., & Peles, E. (2003). The local differentiation of myelinated axons at nodes of Ranvier. Nature Reviews Neuroscience, 4, 968–980.
Powers, B. E., Lasiene, J., Plemel, J. R., Shupe, L., Perlmutter, S. I., Tetzlaff, W., & Horner, P. J. (2012). Axonal thinning and extensive remyelination without chronic demyelination in spinal injured rats. Journal of Neuroscience, 32, 5120–5125.
Robertson, J. D. (1959). Preliminary observations on the ultrastructure of nodes of Ranvier. Zeitschrift fur Zellforschung und Mikroscopische Anatomie, 50, 553–560.
Roots, B. I. (2008). The phylogeny of invertebrates and the evolution of myelin. Neuron Glia Biology, 4, 101–109.
Rovainen, C. M. (1967). Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Müller and Mauthner cells. Journal of Neurophysiology, 30, 1000–1023.
Rushton, W. A. H. (1951). A theory of the effects of fibre size in medullated nerve. Journal of Physiology, 115, 101–122.
Sangrey, T. D., Friesen, W. O., & Levy, W. B. (2004). Analysis of the optimal channel density of the giant squid axon using a reparameterized Hodgkin-Huxley model. Journal of Neurophysiology, 91, 2541–2550.
Schweigreiter, R., Roots, B. I., Bandtlow, C. E., & Gould, R. M. (2006). Understanding myelination through studying its evolution. International Review of Neurobiology, 73, 219–273.
Simpson, A. H., Gillingwater, T. H., Anderson, H., Cottrell, D., Sherman, D. L., Ribchester, R. R., & Brophy, P. J. (2013). Effect of limb lengthening on internodal length and conduction of peripheral nerve. Journal of Neuroscience, 33, 4536–4539.
Smith, R. S., & Koles, Z. J. (1970). Myelinated nerve fibers: computed effect of myelin thickness on conduction velocity. American Journal of Physiology, 219, 1256–1258.
Snaidero, N., Möbius, W., Czopka, T., Hekking, L. H. P., Mathisen, C., Verkleij, D., Goebbels, S., Edgar, J., Merkler, D., Lyons, D. A., Nave, K.-A., & Simons, M. (2014). Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell, 156, 277–290.
Tomassy, G. S., Berger, D. R., Chen, H.-H., Kasthuri, N., Hayworth, K. J., Vercelli, A., Seung, H. S., Lichtman, J. W., & Arlotta, P. (2014). Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science, 344, 319–324.
Vabnick, I., & Shrager, P. (1998). Ion channel redistribution and function during development of the myelinated axon. Journal of Neurobiology, 37, 80–96.
Weatherby, T. M., Davis, A. D., Hartline, D. K., & Lenz, P. H. (2000). The need for speed. II. Myelin in calanoid copepods. Journal of Comparative Physiology, 186, 347–357.
Wilson, C. H., & Hartline, D. K. (2011). Novel organization and development of copepod myelin. I. Ontogeny. Journal of Comparative Neurology, 519, 3259–3280.
Wu, L. M. N., Williams, A., Delaney, A., Sherman, D. L., & Brophy, P. J. (2012). Increasing internodal distance in myelinated nerves accelerates nerve conduction to a flat maximum. Current Biology, 22, 1957–1961.
Xu, K., Sun, H., & Sung, Y. (1994). Electronmicroscopic observation on ontogenesis of the myelinated nerve fiber in the shrimp (Panaeus orientalis). Chinese Journal of Neuroanatomy, 10, 239–241.
Young, K. M., Psachoulia, K., Tripathi, R. B., Dunn, S. J., Cossell, L., Attwell, D., Tohyama, K., & Richardson, W. D. (2013a). Oligodendrocyte dynamics in healthy adult CNS: Evidence for myelin remodeling. Neuron, 77, 873–885.
Young, R. G., Castelfranco, A. M., & Hartline, D. K. (2013b). The “Lillie Transition”: models of the onset of saltatory conduction in myelinating axons. Journal of Computational Neuroscience, 34, 533–546.
Acknowledgments
We are grateful to Drs. Michael Hines and Ted Carnevale for the NEURON simulation software and assistance in its application. This work was supported by National Science Foundation grant IOS-0923692 and by the Cades Foundation, Honolulu HI.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Catherine E Carr
Rights and permissions
About this article
Cite this article
Castelfranco, A.M., Hartline, D.K. The evolution of vertebrate and invertebrate myelin: a theoretical computational study. J Comput Neurosci 38, 521–538 (2015). https://doi.org/10.1007/s10827-015-0552-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-015-0552-x