Abstract
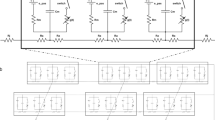
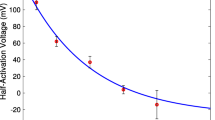
The detrusor, a key component of the urinary bladder wall, is a densely innervated syncytial smooth muscle tissue. Random spontaneous release of neurotransmitter at neuromuscular junctions (NMJs) in the detrusor gives rise to spontaneous excitatory junction potentials (SEJPs). These sub-threshold passive signals not only offer insights into the syncytial nature of the tissue, their spatio-temporal integration is critical to the generation of spontaneous neurogenic action potentials which lead to focal contractions during the filling phase of the bladder. Given the structural complexity and the contractile nature of the tissue, electrophysiological investigations on spatio-temporal integration of SEJPs in the detrusor are technically challenging. Here we report a biophysically constrained computational model of a detrusor syncytium overlaid with spatially distributed innervation, using which we explored salient features of the integration of SEJPs in the tissue and the key factors that contribute to this integration. We validated our model against experimental data, ascertaining that observations were congruent with theoretical predictions. With the help of comparative studies, we propose that the amplitude of the spatio-temporally integrated SEJP is most sensitive to the inter-cellular coupling strength in the detrusor, while frequency of observed events depends more strongly on innervation density. An experimentally testable prediction arising from our study is that spontaneous release frequency of neurotransmitter may be implicated in the generation of detrusor overactivity. Set against histological observations, we also conjecture possible changes in the electrical activity of the detrusor during pathology involving patchy denervation. Our model thus provides a physiologically realistic, heuristic framework to investigate the spread and integration of passive potentials in an innervated syncytial tissue under normal conditions and in pathophysiology.















Similar content being viewed by others
References
Andersson, K. E., Chapple, C., & Wein, A. (2001). The basis for drug treatment of the overactive bladder. World Journal of Urology, 19(5), 294–298.
Appukuttan, S., Brain, K. L., & Manchanda, R. (2015). A computational model of urinary bladder smooth muscle syncytium. Journal of Computational Neuroscience, 38(1), 167–187.
Bennett, M. R. (1973). Structure and electrical properties of the autonomic neuromuscular junction. Philosophical Transactions of the Royal Society of London. B, Biological Sciences, 265(867), 25–34.
Brading, A. F. (1997). A myogenic basis for the overactive bladder. Urology, 50(6), 57–67.
Brading, A. F. (2005). Overactive bladder: Why it occurs. Women's Health Medicine, 2(6), 20–23.
Bramich, N. J., & Brading, A. F. (1996). Electrical properties of smooth muscle in the Guinea-pig urinary bladder. The Journal of Physiology, 492(1), 185–198.
Cash, S., & Yuste, R. (1998). Input summation by cultured pyramidal neurons is linear and position-independent. Journal of Neuroscience, 18(1), 10–15.
Cash, S., & Yuste, R. (1999). Linear summation of excitatory inputs by CA1 pyramidal neurons. Neuron, 22(2), 383–394.
Crane, G. J., Hines, M. L., & Neild, T. O. (2001). Simulating the spread of membrane potential changes in arteriolar networks. Microcirculation, 8(1), 33–43.
Drake, M. J., Gardner, B. P., & Brading, A. F. (2003). Innervation of the detrusor muscle bundle in neurogenic detrusor overactivity. BJU International, 91(7), 702–710.
Drake, M. J., Kanai, A., Bijos, D. A., Ikeda, Y., Zabbarova, I., Vahabi, B., & Fry, C. H. (2017). The potential role of unregulated autonomous bladder micromotions in urinary storage and voiding dysfunction; overactive bladder and detrusor underactivity. BJU International, 119(1), 22–29.
Fry, C. H., Cooklin, M., Birns, J., & Mundy, A. R. (1999). Measurement of intercellular electrical coupling in Guinea-pig detrusor smooth muscle. The Journal of Urology, 161(2), 660–664.
Fry, C. H., Sui, G. P., Severs, N. J., & Wu, C. (2004). Spontaneous activity and electrical coupling in human detrusor smooth muscle: Implications for detrusor overactivity? Urology, 63(3), 3–10.
Gabella, G. (1995). The structural relations between nerve fibres and muscle cells in the urinary bladder of the rat. Journal of Neurocytology, 24(3), 159–187.
Gabella, G. (2012). Cells of visceral smooth muscles. Journal of Smooth Muscle Research, 48(4), 65–95.
Goto, K., Millecchia, L. L., Westfall, D. P., & Fleming, W. W. (1977). A comparison of the electrical properties and morphological characteristics of the smooth muscle of the rat and Guinea-pig vas deferens. Pflügers Archiv, 368(3), 253–261.
Hashitani, H. B. N. J., Bramich, N. J., & Hirst, G. D. S. (2000). Mechanisms of excitatory neuromuscular transmission in the Guinea-pig urinary bladder. The Journal of Physiology, 524(2), 565–579.
Hashitani, H., Fukuta, H., Takano, H., Klemm, M. F., & Suzuki, H. (2001). Origin and propagation of spontaneous excitation in smooth muscle of the Guinea-pig urinary bladder. The Journal of Physiology, 530(2), 273–286.
Hashitani, H., Brading, A. F., & Suzuki, H. (2004a). Correlation between spontaneous electrical, calcium and mechanical activity in detrusor smooth muscle of the Guinea-pig bladder. British Journal of Pharmacology, 141(1), 183–193.
Hashitani, H., Yanai, Y., & Suzuki, H. (2004b). Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the Guinea-pig urinary bladder. The Journal of Physiology, 559(2), 567–581.
Hayase, M., Hashitani, H., Kohri, K., & Suzuki, H. (2009). Role of K+ channels in regulating spontaneous activity in detrusor smooth muscle in situ in the mouse bladder. The Journal of Urology, 181(5), 2355–2365.
Inoue, R., & Brading, A. F. (1990). The properties of the ATP-induced depolarization and current in single cells isolated from the Guinea-pig urinary bladder. British Journal of Pharmacology, 100(3), 619–625.
Johnston, L., Cunningham, R. M., Young, J. S., Fry, C. H., McMurray, G., Eccles, R., & McCloskey, K. D. (2012). Altered distribution of interstitial cells and innervation in the rat urinary bladder following spinal cord injury. Journal of Cellular and Molecular Medicine, 16(7), 1533–1543.
Krasnoperov, V. G., Bittner, M. A., Beavis, R., Kuang, Y., Salnikow, K. V., Chepurny, O. G., Little, A. R., Plotnikov, A. N., Wu, D., Holz, R. W., & Petrenko, A. G. (1997). α-Latrotoxin stimulates exocytosis by the interaction with a neuronal G-protein-coupled receptor. Neuron, 18(6), 925–937.
Mahapatra, C., Brain, K. L., & Manchanda, R. (2018). A biophysically constrained computational model of the action potential of mouse urinary bladder smooth muscle. PLoS One, 13(7), e0200712.
Manchanda, R. (1995). Membrane current and potential change during neurotransmission in smooth muscle. Current Science, 69(2), 140–150.
Manchanda, R., Appukuttan, S., & Padmakumar, M. (2019). Electrophysiology of syncytial smooth muscle. Journal of Experimental Neuroscience, 13, 1179069518821917.
Meng, E., Young, J. S., & Brading, A. F. (2008). Spontaneous activity of mouse detrusor smooth muscle and the effects of the urothelium. Neurourology and Urodynamics: Official Journal of the International Continence Society, 27(1), 79–87.
Neuhaus, J., Wolburg, H., Hermsdorf, T., Stolzenburg, J.-U., & Dorschner, W. (2002). Detrusor smooth muscle cells of the Guinea-pig are functionally coupled via gap junctions in situ and in cell culture. Cell and Tissue Research, 309(2), 301–311.
Padmakumar, M., Brain, K. L., Young, J. S., & Manchanda, R. (2018). A four-component model of the action potential in mouse detrusor smooth muscle cell. PLoS One, 13(1), e0190016.
Palani, D., & Manchanda, R. (2006). Effect of heptanol on noradrenaline-induced contractions in rat vas deferens. Journal of Smooth Muscle Research, 42(1), 49–61.
Palani, D., Ghildyal, P., & Manchanda, R. (2006). Effects of carbenoxolone on syncytial electrical properties and junction potentials of Guinea-pig vas deferens. Naunyn-Schmiedeberg's Archives of Pharmacology, 374(3), 207–214.
Poirazi, P., Brannon, T., & Mel, B. W. (2003). Arithmetic of subthreshold synaptic summation in a model CA1 pyramidal cell. Neuron, 37(6), 977–987.
Sengupta, N., Brain, K. L., & Manchanda, R. (2015, August). Spatiotemporal dynamics of synaptic drive in urinary bladder syncytium: A computational investigation. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 8074–8077). IEEE.
Sengupta, N., Brain, L. K., & Manchanda, R. (2018, July). Cellular Environment in a Bundle Modulates SEJP Characteristics in Detrusor Smooth Muscle. In 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 5842–5845). IEEE.
Sourav, S., & Manchanda, R. (2000). Influence of the size of syncytial units on synaptic potentials in smooth muscle. Medical and Biological Engineering and Computing, 38(3), 356–359.
Tomita, T. (1976). Electrophysiology of mammalian smooth muscle. Progress in Biophysics and Molecular Biology, 30, 185–203.
Wang, H. Z., Brink, P. R., & Christ, G. J. (2006). Gap junction channel activity in short-term cultured human detrusor myocyte cell pairs: Gating and unitary conductances. American Journal of Physiology-Cell Physiology, 291(6), C1366–C1376.
Wüst, M., Averbeck, B., Reif, S., Bräter, M., & Ravens, U. (2002). Different responses to drugs against overactive bladder in detrusor muscle of pig, Guinea pig and mouse. European Journal of Pharmacology, 454(1), 59–69.
Young, J. S., Brain, K. L., & Cunnane, T. C. (2007). The origin of the skewed amplitude distribution of spontaneous excitatory junction potentials in poorly coupled smooth muscle cells. Neuroscience, 145(1), 153–161.
Young, J. S., Meng, E., Cunnane, T. C., & Brain, K. L. (2008). Spontaneous purinergic neurotransmission in the mouse urinary bladder. The Journal of Physiology, 586(23), 5743–5755.
Acknowledgements
The work was supported by grants from the Department of Biotechnology (DBT), India (BT/PR12973/MED/122/47/2016) and the UK-India Education and Research Initiative, UKIERI (UKUTP20110055).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Action Editor: Upinder Singh Bhalla
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sengupta, N., Manchanda, R. Spontaneous synaptic drive in detrusor smooth muscle: computational investigation and implications for urinary bladder function. J Comput Neurosci 47, 167–189 (2019). https://doi.org/10.1007/s10827-019-00731-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-019-00731-7