Abstract
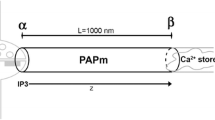
Information transfer may not be limited only to synapses. Therefore, the processes and dynamics of biological neuron-astrocyte coupling and intercellular interaction within this domain are worth investigating. Existing models of tripartite synapse consider an astrocyte as a point process. Here, we extended the tripartite synapse model by considering the astrocytic processes (synaptic and perinodal) as compartments. The scattered extrinsic signals in the extracellular space and the presence of calcium stores in different astrocytic sites create local transient [Ca2+]. We investigated the Ca2+ dynamics and found that the increase in astrocytic intracellular [Ca2+] enhances the probability of neurotransmitter release. However, the period in which the extrasynaptic glutamate lingers in the extracellular space may cause excitotoxicity. We propose further biological investigation on intercellular communication, considering that unconventional sources (nonsynaptic) of glutamate may improve information processing in neuron-astrocyte networks.







Similar content being viewed by others
References
Abbracchio, M.P., Burnstock, G., Verkhratsky, A., Zimmermann, H. (2009). Purinergic signalling in the nervous system: an overview. Trends in Neurosciences, 32(1), 19–29.
Amiri, M., Hosseinmardi, N., Bahrami, F., Janahmadi, M. (2013). Astrocyte-neuron interaction as a mechanism responsible for generation of neural synchrony: a study based on modeling and experiments. Journal of Computational Neuroscience, 34(3), 489–504.
Arancibia-Carcamo, I.L., Ford, M.C., Cossell, L., Ishida, K., Tohyama, K., Attwell, D. (2017). Node of ranvier length as a potential regulator of myelinated axon conduction speed. Elife, 6, e23,329.
Ashhad, S., & Narayanan, R. (2018). Stores, channels, glue, and trees: active glial and active dendritic physiology. Molecular Neurobiology, 1–22.
Babbs, C.F., & Shi, R. (2013). Subtle paranodal injury slows impulse conduction in a mathematical model of myelinated axons. PLoS One, 8(7), e67,767.
Barros, M, & Dey, S. (2018). Feed-forward and feedback control in astrocytes for ca2+-based molecular communications nanonetworks. IEEE/ACM Transactions on Computational Biology and Bioinformatics.
Bazargani, N., & Attwell, D. (2016). Astrocyte calcium signaling: the third wave. Nature Neuroscience, 19 (2), 182.
Bezzi, P., & Volterra, A. (2014). Imaging exocytosis and recycling of synaptic-like microvesicles in astrocytes. Cold Spring Harbor Protocols, 2014(5), pdb–prot081,711.
Bogatov, N., Grigoryan, L., Ponetaeva, E., Sinisyn, A. (2014). Calculation of action potential propagation in nerve fiber. Progress in Biophysics and Molecular Biology, 114(3), 170–174.
Bucher, D., & Goaillard, J.M. (2011). Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Progress in Neurobiology, 94(4), 307–346.
Bushong, E.A., Martone, M.E., Jones, Y.Z., Ellisman, M.H. (2002). Protoplasmic astrocytes in ca1 stratum radiatum occupy separate anatomical domains. Journal of Neuroscience, 22(1), 183–192.
Bushong, E.A., Martone, M.E., Ellisman, M.H. (2004). Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. International Journal of Developmental Neuroscience, 22(2), 73–86.
Butt, A.M. (2011). Atp: a ubiquitous gliotransmitter integrating neuron–glial networks. In Seminars in cell & developmental biology, (Vol. 22 pp. 205–213): Elsevier.
Chan, S.C., Mok, S.Y., Ng, D.W.K., Goh, S.Y. (2017). The role of neuron–glia interactions in the emergence of ultra-slow oscillations. Biological Cybernetics, 111(5–6), 459–472.
Choi, M., Ahn, S., Yang, E.J., Kim, H., Chong, Y.H., Kim, H.S. (2016). Hippocampus-based contextual memory alters the morphological characteristics of astrocytes in the dentate gyrus. Molecular Brain, 9(1), 72.
Cinciute, S. (2019). Translating the hemodynamic response: why focused interdisciplinary integration should matter for the future of functional neuroimaging. PeerJ, 7, e6621.
de Juan-Sanz, J., Holt, G.T., Schreiter, E.R., de Juan, F., Kim, D.S., Ryan, T.A. (2017). Axonal endoplasmic reticulum ca 2+ content controls release probability in cns nerve terminals. Neuron, 93(4), 867–881.
De Pittà, M, & Brunel, N. (2016). Modulation of synaptic plasticity by glutamatergic gliotransmission: a modeling study. Neural Plasticity, 2016.
De Pittà, M, Brunel, N., Volterra, A. (2016). Astrocytes: orchestrating synaptic plasticity? Neuroscience, 323, 43–61.
Debanne, D, & Rama, S. (2011). Astrocytes shape axonal signaling. Science of Signal, 4(162), pe11–pe11.
Del-Bel, E., & De-Miguel, F.F. (2018). Extrasynaptic neurotransmission mediated by exocytosis and diffusive release of transmitter substances. Frontiers in Synaptic Neuroscience, 10.
Deplanque, D. (2009). Maladie d’alzheimer: dualité des effets physiologiques et pathologiques du glutamate. La Lettre du pharmacologue Supplé,ment, 23(4), 13–22.
Ding, X., Zhang, X., Ji, L. (2018). Contribution of calcium fluxes to astrocyte spontaneous calcium oscillations in deterministic and stochastic models. Applied Mathematical Modelling, 55, 371–382.
Durkee, C.A., & Araque, A. (2018). Diversity and specificity of astrocyte-neuron communication. Neuroscience.
Dutta, D.J., Woo, D.H., Lee, P.R., Pajevic, S., Bukalo, O., Huffman, W.C., Wake, H., Basser, P.J., SheikhBahaei, S., Lazarevic, V., et al. (2018). Regulation of myelin structure and conduction velocity by perinodal astrocytes. Proceedings of the National Academy of Sciences, 115(46), 11,832–11,837.
English, D.F., McKenzie, S., Evans, T., Kim, K., Yoon, E., Buzsáki, G. (2017). Pyramidal cell-interneuron circuit architecture and dynamics in hippocampal networks. Neuron, 96(2), 505–520.
Evans, R., & Blackwell, K. (2015). Calcium: amplitude, duration, or location? The Biological Bulletin, 228 (1), 75–83.
Fiacco, T.A., & McCarthy, K.D. (2018). Multiple lines of evidence indicate that gliotransmission does not occur under physiological conditions. Journal of Neuroscience, 38(1), 3–13.
Fletcher, A. (2016). Nerve cell function and synaptic mechanisms. Anaesthesia & Intensive Care Medicine, 17 (4), 199–203.
Ford, M.C., Alexandrova, O., Cossell, L., Stange-Marten, A., Sinclair, J., Kopp-Scheinpflug, C., Pecka, M., Attwell, D., Grothe, B. (2015). Tuning of ranvier node and internode properties in myelinated axons to adjust action potential timing. Nature Communications, 6, 8073.
Freeman, S.A., Desmazieres, A., Fricker, D., Lubetzki, C., Sol-Foulon, N. (2016). Mechanisms of sodium channel clustering and its influence on axonal impulse conduction. Cellular and Molecular Life Sciences, 73 (4), 723–735.
Genç, Ö., Dickman, D.K., Ma, W., Tong, A., Fetter, R.D., Davis, G.W. (2017). Mctp is an er-resident calcium sensor that stabilizes synaptic transmission and homeostatic plasticity. Elife, 6, e22,904.
Gordleeva, S.Y., Stasenko, S.V., Semyanov, A.V., Dityatev, A.E., Kazantsev, V.B. (2012). Bi-directional astrocytic regulation of neuronal activity within a network. Frontiers in Computational Neuroscience, 6, 92.
Gordleeva, S.Y., Lebedev, S., Rumyantseva, M., Kazantsev, V.B. (2018). Astrocyte as a detector of synchronous events of a neural network. JETP Letters, 107(7), 440–445.
Graupner, M., & Brunel, N. (2010). Mechanisms of induction and maintenance of spike-timing dependent plasticity in biophysical synapse models. Frontiers in Computational Neuroscience, 4, 136.
Guerra-Gomes, S., Sousa, N., Pinto, L., Oliveira, J.F. (2018). Functional roles of astrocyte calcium elevations: from synapses to behavior. Frontiers in Cellular Neuroscience, 11, 427.
Gulledge, A.T., & Bravo, J.J. (2016). Neuron morphology influences axon initial segment plasticity. eNeuro, ENEURO–0085.
Guo, Y., Liu, Z., Yk, Chen, Chai, Z., Zhou, C., Zhang, Y. (2017). Neurons with multiple axons have functional axon initial segments. Neuroscience Bulletin, 33(6), 641–652.
Halassa, M.M., Fellin, T., Takano, H., Dong, J.H., Haydon, P.G. (2007). Synaptic islands defined by the territory of a single astrocyte. Journal of Neuroscience, 27(24), 6473–6477.
Handy, G., Taheri, M., White, J.A., Borisyuk, A. (2017). Mathematical investigation of ip 3-dependent calcium dynamics in astrocytes. Journal of Computational Neuroscience, 42(3), 257–273.
Heller, J.P., & Rusakov, D.A. (2017). The nanoworld of the tripartite synapse: insights from super-resolution microscopy. Frontiers in Cellular Neuroscience, 11, 374.
Hennig, M.H. (2013). Theoretical models of synaptic short term plasticity. Frontiers in Computational Neuroscience, 7, 45.
Hliatsevich, M.A., Bulai, P.M., Pitlik, T.N., Denisov, A.A., Cherenkevich, S.N. (2015). Design of deterministic model of signal transduction between neuronal cells. Mathematical Modelling and Analysis, 20(1), 76–93.
Hu, X., Yuan, Y., Wang, D., Su, Z. (2016). Heterogeneous astrocytes: active players in cns. Brain Research Bulletin, 125, 1–18.
Jourdain, P., Bergersen, L.H., Bhaukaurally, K., Bezzi, P., Santello, M., Domercq, M., Matute, C., Tonello, F., Gundersen, V., Volterra, A. (2007). Glutamate exocytosis from astrocytes controls synaptic strength. Nature Neuroscience, 10(3), 331.
Kelso, J.S., Dumas, G., Tognoli, E. (2013). Outline of a general theory of behavior and brain coordination. Neural Networks, 37, 120–131.
Kettenmann, H., & Verkhratsky, A. (2008). Neuroglia: the 150 years after. Trends in Neurosciences, 31(12), 653–659.
Kole, M.H. (2011). First node of ranvier facilitates high-frequency burst encoding. Neuron, 71(4), 671–682.
Kole, M.H., & Brette, R. (2018). The electrical significance of axon location diversity. Current Opinion in Neurobiology, 51, 52–59.
Kuznetsov, I, & Kuznetsov, A. (2017). How dense core vesicles are delivered to axon terminals–a review of modeling approaches. In Modeling of microscale transport in biological processes (pp. 335–352). Elsevier.
Li, J.J., Du, M.M., Wang, R., Lei, J.Z., Wu, Y. (1650). Astrocytic gliotransmitter: diffusion dynamics and induction of information processing on tripartite synapses. International Journal of Bifurcation and Chaos, 26 (08), 138.
London, M., Schreibman, A., Häusser, M, Larkum, M.E., Segev, I. (2002). The information efficacy of a synapse. Nature Neuroscience, 5(4), 332.
López-Caamal, F, Oyarzún, D A, Middleton, R.H., García, M.R. (2014). Spatial quantification of cytosolic ca 2+ accumulation in nonexcitable cells: an analytical study. IEEE/ACM Transactions on Computational Biology and Bioinformatics (TCBB), 11(3), 592–603.
Manninen, T., Havela, R., Linne, M.L. (2018). Computational models for calcium-mediated astrocyte functions. Frontiers in Computational Neuroscience, 12, 14.
Manninen, T., Havela, R., Linne, M.L. (2019). Computational models of astrocytes and astrocyte–neuron interactions: characterization, reproducibility, and future perspectives. In Computational glioscience (pp. 423–454): Springer.
Mirzakhalili, E., Epureanu, B.I., Gourgou, E. (2018). A mathematical and computational model of the calcium dynamics in caenorhabditis elegans ash sensory neuron. PloS One, 13(7), e0201,302.
Mitterauer, B.J. (2014). Pathophysiology of schizophrenia based on impaired glial-neuronal interactions. Open Journal of Medical Psychology, 3(02), 126.
Modchang, C., Nadkarni, S., Bartol, T.M., Triampo, W., Sejnowski, T.J., Levine, H., Rappel, W.J. (2010). A comparison of deterministic and stochastic simulations of neuronal vesicle release models. Physical Biology, 7(2), 026,008.
Namazi, H., & Kulish, V.V. (2013). A mathematical based calculation of a myelinated segment in axons. Computers in Biology and Medicine, 43(6), 693–698.
Nazari, S., Faez, K., Amiri, M., Karami, E. (2015). A digital implementation of neuron–astrocyte interaction for neuromorphic applications. Neural Networks, 66, 79–90.
Nedergaard, M., & Verkhratsky, A. (2012). Artifact versus reality—how astrocytes contribute to synaptic events. Glia, 60(7), 1013–1023.
Nelson, A.D., & Jenkins, P.M. (2017). Axonal membranes and their domains: assembly and function of the axon initial segment and node of ranvier. Frontiers in Cellular Neuroscience, 11, 136.
of Notre Dame, U. (2004). The electrical system of the body. In Physics in medicine (pp. 224–242): Elsevier.
Perea, G., Sur, M., Araque, A. (2014). Neuron-glia networks: integral gear of brain function. Frontiers in Cellular Neuroscience, 8, 378.
Pissadaki, E.K., Sidiropoulou, K., Reczko, M., Poirazi, P. (2010). Encoding of spatio-temporal input characteristics by a ca1 pyramidal neuron model. PLoS Computational Biology, 6(12), e1001,038.
Poliak, S., & Peles, E. (2003). The local differentiation of myelinated axons at nodes of ranvier. Nature Reviews Neuroscience, 4(12), 968.
Robertson, J.M. (2013). Astrocyte domains and the three-dimensional and seamless expression of consciousness and explicit memories. Medical Hypotheses, 81(6), 1017–1024.
Rossi, D. (2015). Astrocyte physiopathology: at the crossroads of intercellular networking, inflammation and cell death. Progress in Neurobiology, 130, 86–120.
Sasaki, T. (2013). The axon as a unique computational unit in neurons. Neuroscience Research, 75(2), 83–88.
Sasaki, T., Matsuki, N., Ikegaya, Y. (2011). Action-potential modulation during axonal conduction. Science, 331(6017), 599–601.
Savtchouk, I., & Volterra, A. (2018). Gliotransmission: beyond black-and-white. Journal of Neuroscience, 38 (1), 14–25.
Semyanov, A. (2018). Spatiotemporal pattern of ca2+ activity in astrocytic network. Cell Calcium.
Shigetomi, E., Patel, S., Khakh, B.S. (2016). Probing the complexities of astrocyte calcium signaling. Trends in Cell Biology, 26(4), 300–312.
Sims, R.E., Butcher, J.B., Parri, H.R., Glazewski, S. (2015). Astrocyte and neuronal plasticity in the somatosensory system. Neural Plasticity, 2015.
Sloan, S.A., & Barres, B.A. (2014). Looks can be deceiving: reconsidering the evidence for gliotransmission. Neuron, 84(6), 1112–1115.
Sosunov, A.A., Wu, X., Tsankova, N.M., Guilfoyle, E., McKhann, G.M., Goldman, J.E. (2014). Phenotypic heterogeneity and plasticity of isocortical and hippocampal astrocytes in the human brain. Journal of Neuroscience, 34(6), 2285–2298.
Tewari, S., & Parpura, V. (2013). A possible role of astrocytes in contextual memory retrieval: an analysis obtained using a quantitative framework. Frontiers in Computational Neuroscience, 7, 145.
Tewari, S.G., & Majumdar, K.K. (2012). A mathematical model of the tripartite synapse: astrocyte-induced synaptic plasticity. Journal of Biological Physics, 38(3), 465–496.
Tønnesen, J, & Nägerl, U.V. (2016). Dendritic spines as tunable regulators of synaptic signals. Frontiers in Psychiatry, 7, 101.
Trueta, C., & De-Miguel, F.F. (2012). Extrasynaptic exocytosis and its mechanisms: a source of molecules mediating volume transmission in the nervous system. Frontiers in Physiology, 3, 319.
Ventura, R., & Harris, K.M. (1999). Three-dimensional relationships between hippocampal synapses and astrocytes. Journal of Neuroscience, 19(16), 6897–6906.
Verkhratsky, A., Matteoli, M., Parpura, V., Mothet, J.P., Zorec, R. (2016). Astrocytes as secretory cells of the central nervous system: idiosyncrasies of vesicular secretion. The EMBO Journal, 35(3), 239–257.
Vizi, E.S., & Kiss, J.P. (1998). Neurochemistry and pharmacology of the major hippocampal transmitter systems: synaptic and nonsynaptic interactions. Hippocampus, 8(6), 566–607.
Volterra, A., & Meldolesi, J. (2005). Astrocytes, from brain glue to communication elements: the revolution continues. Nature Reviews Neuroscience, 6(8), 626.
Volterra, A., Liaudet, N., Savtchouk, I. (2014). Astrocyte ca 2+ signalling: an unexpected complexity. Nature Reviews Neuroscience, 15(5), 327.
Wade, J.J., McDaid, L.J., Harkin, J., Crunelli, V., Kelso, J.S. (2011). Bidirectional coupling between astrocytes and neurons mediates learning and dynamic coordination in the brain: a multiple modeling approach. PloS One, 6(12), e29,445.
Wallach, G., Lallouette, J., Herzog, N., De Pittà, M, Jacob, E.B., Berry, H., Hanein, Y. (2014). Glutamate mediated astrocytic filtering of neuronal activity. PLoS Computational Biology, 10(12), e1003,964.
Woo, B., & Choi, J. (2007). Reduced model and simulation of myelinated axon using eigenfunction expansion and singular perturbation. Computers in Biology and Medicine, 37(8), 1148–1154.
Wu, Y.W., Tang, X., Arizono, M., Bannai, H., Shih, P.Y., Dembitskaya, Y., Kazantsev, V., Tanaka, M., Itohara, S., Mikoshiba, K., et al. (2014). Spatiotemporal calcium dynamics in single astrocytes and its modulation by neuronal activity. Cell Calcium, 55(2), 119–129.
Wu, Y.W., Gordleeva, S., Tang, X., Shih, P.Y., Dembitskaya, Y., Semyanov, A. (2018). Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia.
Yamada, R., & Kuba, H. (2016). Structural and functional plasticity at the axon initial segment. Frontiers in Cellular Neuroscience, 10, 250.
Ye, H., & Ng, J. (2018). Shielding effects of myelin sheath on axolemma depolarization under transverse electric field stimulation. PeerJ, 6, e6020.
Zbili, M., Rama, S., Debanne, D. (2016). Dynamic control of neurotransmitter release by presynaptic potential. Frontiers in Cellular Neuroscience, 10, 278.
Zhou, B., Zuo, Y.X., Jiang, R.T. (2019). Astrocyte morphology: diversity, plasticity, and role in neurological diseases. CNS Neuroscience & Therapeutics, 25(6), 665–673.
Ziskin, J.L., Nishiyama, A., Rubio, M., Fukaya, M., Bergles, D.E. (2007). Vesicular release of glutamate from unmyelinated axons in white matter. Nature Neuroscience, 10(3), 321.
Acknowledgements
The authors would also like to acknowledge Shivendra G. Tewari and Kaushik Kumar Majumdar for sharing the Matlab code essential for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Additional information
Action Editor: Upinder Singh Bhalla
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. Lorenzo would like to thank the CHED-PhilFrance for the scholarship grant.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lorenzo, J., Vuillaume, R., Binczak, S. et al. Spatiotemporal model of tripartite synapse with perinodal astrocytic process. J Comput Neurosci 48, 1–20 (2020). https://doi.org/10.1007/s10827-019-00734-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-019-00734-4