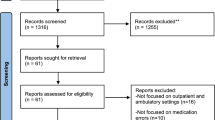
Abstract
Commonly used drugs in hospital setting can cause QT prolongation and trigger life-threatening arrhythmias. We evaluate changes in prescribing behavior after the implementation of a clinical decision support system to prevent the use of QT prolonging medications in the hospital setting. We conducted a quasi-experimental study, before and after the implementation of a clinical decision support system integrated in the electronic medical record (QT-alert system). This system detects patients at risk of significant QT prolongation (QTc>500ms) and alerts providers ordering QT prolonging drugs. We reviewed the electronic health record to assess the provider’s responses which were classified as “action taken” (QT drug avoided, QT drug changed, other QT drug(s) avoided, ECG monitoring, electrolytes monitoring, QT issue acknowledged, other actions) or “no action taken”. Approximately, 15.5% (95/612) of the alerts were followed by a provider’s action in the pre-intervention phase compared with 21% (228/1085) in the post-intervention phase (p=0.006). The most common type of actions taken during pre-intervention phase compared to post-intervention phase were ECG monitoring (8% vs. 13%, p=0.002) and QT issue acknowledgment (2.1% vs. 4.1%, p=0.03). Notably, there was no significant difference for other actions including QT drug avoided (p=0.8), QT drug changed (p=0.06) and other QT drug(s) avoided (p=0.3). Our study demonstrated that the QT alert system prompted a higher proportion of providers to take action on patients at risk of complications. However, the overall impact was modest underscoring the need for educating providers and optimizing clinical decision support to further reduce drug-induced QT prolongation.

Similar content being viewed by others
References
AZCERT, Credible Meds. http://crediblemedsorg/ Accessed Dec 31, 2014. 2014.
Drew, B.J., Ackerman, M.J., Funk, M., et al., Prevention of torsade de pointes in hospital settings: A scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 121(8):1047–1060, 2010.
Priori, S.G., Schwartz, P.J., Napolitano, C., et al., Risk stratification in the long-QT syndrome. N. Engl J Med. 348(19):1866–1874, 2003.
Bednar, M.M., Harrigan, E.P., Anziano, R.J., Camm, A.J., and Ruskin, J.N., The QT interval. Prog Cardiovasc Dis. 43(5 Suppl 1):1–45, 2001.
Golzari, H., Dawson, N.V., Speroff, T., and Thomas, C., Prolonged QTc intervals on admission electrocardiograms: Prevalence and correspondence with admission electrolyte abnormalities. Conn. Med. 71(7):389–397, 2007.
Seftchick, M.W., Adler, P.H., Hsieh, M., et al., The prevalence and factors associated with QTc prolongation among emergency department patients. Ann. Emerg. Med. 54(6):763–768, 2009.
Dumontet, J., Malyuk, R., Kiang, G., and Procyshyn, R.M., Corrected QT intervals in newly admitted geriatric psychiatric patients: An examination of risk factors. Can. J. Psychiatry. 51(6):371–376, 2006.
Lubart, E., Segal, R., Yearovoi, A., Fridenson, A., Baumoehl, Y., and Leibovitz, A., QT interval disturbances in hospitalized elderly patients. Isr Med Assoc J. 11(3):147–150, 2009.
Tisdale, J.E., Wroblewski, H.A., Overholser, B.R., Kingery, J.R., Trujillo, T.N., and Kovacs, R.J., Prevalence of QT interval prolongation in patients admitted to cardiac care units and frequency of subsequent administration of QT interval-prolonging drugs: A prospective, observational study in a large urban academic medical center in the US. Drug Saf. 35(6):459–470, 2012.
Pell, J.M., Cheung, D., Jones, M.A., and Cumbler, E., Don't fuel the fire: Decreasing intravenous haloperidol use in high risk patients via a customized electronic alert. J. Am. Med. Inform. Assoc. 21(6):1109–1112, 2014.
Muzyk, A.J., Rivelli, S.K., Jiang, W., Heinz, H., Rayfield, A., and Gagliardi, J.P., A computerized physician order entry set designed to improve safety of intravenous haloperidol utilization: A retrospective study in agitated hospitalized patients. Drug Saf. 35(9):725–731, 2012.
Kuperman, G.J., Bobb, A., Payne, T.H., et al., Medication-related clinical decision support in computerized provider order entry systems: A review. J. Am. Med. Inform. Assoc. 14(1):29–40, 2007.
Schedlbauer, A., Prasad, V., Mulvaney, C., et al., What evidence supports the use of computerized alerts and prompts to improve clinicians' prescribing behavior? J. Am. Med. Inform. Assoc. 16(4):531–538, 2009.
Sorita A, Bos JM, Morlan BW, Tarrell RF, Ackerman MJ, Caraballo PJ. Impact of clinical decision support preventing the use of QT-prolonging medications for patients at risk for torsade de pointes. J. Am. Med. Inform. Assoc. 2014.
Haugaa, K.H., Bos, J.M., Tarrell, R.F., Morlan, B.W., Caraballo, P.J., and Ackerman, M.J., Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin. Proc. 88(4):315–325, 2013.
Tisdale, J.E., Jaynes, H.A., Kingery, J.R., et al., Effectiveness of a clinical decision support system for reducing the risk of QT interval prolongation in hospitalized patients. Circ. Cardiovasc. Qual. Outcomes. 7(3):381–390, 2014.
Obal, D., Yang, D., and Sessler, D.I., Perioperative doses of ondansetron or dolasetron do not lengthen the QT interval. Mayo Clin. Proc. 89(1):69–80, 2014.
Meyer-Massetti, C., Cheng, C.M., Sharpe, B.A., Meier, C.R., and Guglielmo, B.J., The FDA extended warning for intravenous haloperidol and torsades de pointes: How should institutions respond? J. Hosp. Med. 5(4):E8–16, 2010.
Judge, J., Field, T.S., DeFlorio, M., et al., Prescribers' responses to alerts during medication ordering in the long term care setting. J. Am. Med. Inform. Assoc. 13(4):385–390, 2006.
Nanji, K.C., Slight, S.P., Seger, D.L., et al., Overrides of medication-related clinical decision support alerts in outpatients. J. Am. Med. Inform. Assoc. 21(3):487–491, 2014.
Acknowledgements
This study was supported partially by a generous gift from the Frederick W. Smith family and by the Mayo Clinic Windland Smith Rice Comprehensive Sudden Cardiac Death Program. Its contents are solely the responsibility of the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
Dr. Ackerman is a consultant for Boston Scientific, Gilead Sciences, Invitae, Myokardia, Medtronic, and St. Jude Medical. Dr. Ackerman and Mayo Clinic receive sales based royalties from Transgenomic for their FAMILION-LQTS and FAMILION-CPVT genetic tests. The other authors have no conflicts of interest to disclose. None of the disclosures pertain to this paper and none of the companies provided financial support for this paper.
Additional information
This article is part of the Topical Collection on Systems-Level Quality Improvement
Rights and permissions
About this article
Cite this article
Sharma, S., Martijn Bos, J., Tarrell, R.F. et al. Providers’ Response to Clinical Decision Support for QT Prolonging Drugs. J Med Syst 41, 161 (2017). https://doi.org/10.1007/s10916-017-0803-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10916-017-0803-7