Abstract
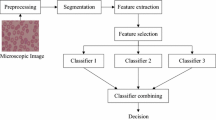
This paper aims to develop the computer assisted malaria infected erythrocyte classification based on a hybrid classifier. The major issues are feature extraction, optimal feature selection and erythrocytes classification. 54 dimensional features formed by the combination of the proposed features and the existing features have been used to define the feature set. The features such as prediction error, co-occurrence of linear binary pattern, chrominance channel histogram, R-G color channel difference histogram are the newly proposed features in our system. For feature selection, the different techniques have been explored to obtain the optimal feature set. Further, the performance of the different individual classifiers (SVM, k-NN and Naive Bayes) and hybrid classifier, obtained by combining the individual classifiers, is evaluated using the optimal feature set. Using the proposed optimal feature set and hybrid model, better performances (i.e. sensitivity 95.86%, accuracy 98.5%, F-score 93.82%) have been achieved on the collected clinical database. Based on the experimental results it may be concluded that hybrid classifier provides satisfactory results with an improvement in sensitivity (1.09%, 12.04%, 0%), accuracy (0.12%, 1.15%, 1.27%) and F-score (0.7%, 5.77%, 4.61%) as compared to the individual classifiers i.e. SVM, k-NN and Naive Bayes respectively.








Similar content being viewed by others
Change history
02 March 2017
An erratum to this article has been published.
References
Abdul-Nasir AS, Mashor MY, Mohamed Z (2013) Colour image segmentation approach for detection of malaria parasites using various colour models and k-means clustering. WSEAS T Biol Biomed 10(1):41–55
Altman NS (1992) An introduction to kernel and nearest-neighbor non parametric regression. Am Stat 46(3):175–185
Annaldas S, Shirgan SS, Marathe VR (2014) Automatic identification of malaria parasites using image processing. Int J Emerg Eng Res Technol 2:107–112
Bairagi VK, Charpe KC (2016) Comparison of texture features used for classification of life stages of malaria parasite. Int J Biomed Imag. Article ID 7214156
Burges CJC (1998) A tutorial on support vector machines for pattern recognition. Data Min Knowl Disc 2(2):121–167
Centers for Diseases Control and Prevention (2013) USA. http://www.cdc.gov/dpdx/malaria/gallery.html. Accessed 21 May 2015
Chandrashekar G, Sahin F (2014) A survey on feature selection methods. Comput Electr Eng 40(1):16–28
Chen W, Lin H, Feng PM, Ding C, Zuo YC, Chou K-C (2012) iNuc-PhysChem: a sequence-based predictor for identifying nucleosomes via physicochemical properties. PLoS One 7(10):e47843. doi:10.1371/journal.pone.0047843
Cheng C, Rajapakse JC (2009) Segmentation of clustered nuclei with shape markers and marking function. IEEE T Biomed Eng 56(3):741–748
Chowdhury S, Verma B, Stockwell D (2015) A novel texture feature based multiple classifier technique for roadside vegetation classification. Expert Syst Appl 42(12):5047–5055
Cortes C, Vapnik V (1995) Support-vector networks. Mach Learn 20(3):273–297
Cuomo MJ, Noel LB, White DB (2012) Diagnosing medical parasites: a public health officers guide to assisting laboratory and medical officers. http://www.phsource.us/PH/PARA/DiagnosingMedicalParasites
Das DK, Ghosh M, Chakraborty C, Maiti AK, Pal M (2011) Probabilistic prediction of malaria using morphological and textural information. In: Proceedings of International Conference on Image InformationProcessing, India
Das DK, Maiti AK, Chakraborty C (2012) Textural pattern classification of microscopic images for malaria screening. Advances in therapeutic engineering. CRC Press, Boca Raton, pp. 419–446
Das DK, Ghosh M, Pal M, Maiti AK, Chakraborty C (2013) Machine learning approach for automated screening of malaria parasite using light microscopic images. Micron 45:97–106
Dash JK, Mukhopadhyay S, Gupta RD (2016) Multiple classifier system using classification confidence for texture classification. Multimed Tools Appl :1–22. doi:10.1007/s11042-015-3231-z
Devi SS, Kumar R, Laskar RH (2015) Recent advances on erythrocyte image segmentation for biomedical applications. In: In: Proceedings of Fourth International Conference on Soft Computing for Problem Solving. Springer, India, pp. 353–359
Dhiman S, Baruah I, Singh L (2010) Military malaria in northeast region of India. Def Sci J 60(2):213–218
Di Ruberto C, Dempster A, Khan S, Jarra B (2002) Analysis of infected blood cell images using morphological operators. Image Vis Comput 20(2):133–146
Diaz G, Gonzalez FA, Romero E (2007) Infected cell identification in thin blood images based on color pixel classification: comparison and analysis. In: Proceedings of Iberoamericann Congress on Pattern Recognition, CIARP, 2007, Springer, pp 812–821
Diaz G, Gonzalez FA, Romero E (2009) A semi-automatic method for quantification and classification of erythrocytes infected with malaria parasites in microscopic images. J Biomed Inform 42(2):296–307
Ding H, Feng PM, Chen W, Lin H (2014) Identification of bacteriophage virion proteins by the ANOVA feature selection and analysis. Mol Bio syst 10(8):2229–2235
Eun S, Kim H, Park J (2015) Effective object segmentation based on physical theory in an MR image. Multimed Tools Appl 74(16):6273–6286
Fan D, Wei L, Cao M (2016) Extraction of target region in lung immunohistochemical image based on artificial neural network. Multimed Tools Appl. 75(19):12227–12244
Ghosh M, Das DK, Chakraborty C, Ray AK (2013) Quantitative characterisation of plasmodium vivax in infected erythrocytes: a textural approach. Int J Artif Intell Soft Co 3(3):203–221
Gitonga L, Memeu DM, Kaduki KA, Kale MAC, Muriuki NS (2014) Determination of plasmodium parasite life stages and species in images of thin blood smears using artificial neural network. Open J Clin Diag 4:78–88
Hahnel M, Klunder D, Kraiss, K-F (2004) Color texture features for person recognition. In Proceedings of IEEE International Joint Conference on Neural Networks 2004, pp 647–652
Jung C, Kim C (2010) Segmenting clustered nuclei using h-minima transform- based marker extraction and contour parameterization. IEEE T Biomed Eng 57(10):2600–2604
Khan MI, Acharya B, Singh BK, Soni J (2011) Content based image retrieval approaches for detection of malaria parasite in blood images. Int J Biom Bioinforma 5(2):97–110
Kumarasamy SK, Ong SH, Tan KSW (2011) Robust contour reconstruction of red blood cells and parasites in the automated identification of the stages of malaria infection. Mach Vis Appl 22(3):461–469
Kuncheva LI (2004) Combining pattern classifiers: methods and algorithms, 2nd edn. Wiley, New Jersey
Maity M, Maiti AK, Dutta PK, Chakraborty C (2012) A web accessible framework for automated storage with compression and textural classification of malaria parasite images. Int J Comput Appl 52(15):31–39
Mejdoub M, Amar CB (2013) Classification improvement of local feature vectors over the k-NN algorithm. Multimed Tools Appl. 64(1):197–218
Memeu DM (2014) A rapid malaria diagnostic method based on automatic detection and classification of plasmodium parasites in stained thin blood smear images. Doctoral dissertation, University of Nairobi
Moore AW (2001) Cross-validation for detecting and preventing overfitting. School of Computer Science Carneigie. Mellon University
Murphy SC, Shott JP, Parikh S, Etter P, Prescott WR, Stewart VA (2013) Review article: malaria diagnostics in clinical trials. AmJTrop Med Hyg 89(5):824–839
Nicholas RE, Charles JP, David MR, Adriano GD (2006) Automated image processing method for the diagnosis and classification of malaria on thin blood smears. Med Biol Eng Comput 44(5):427–436
Niu B, Huang G, Zheng L, Wang X, Chen F, Zhang Y, Huang T (2012) Prediction of substrate-enzyme-product interaction based on molecular descriptors and physicochemical properties. BioMed Res Int. Article ID 674215
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE T Sys Man and Cyber 9(1):62–66
Prasad K, Winter J, Bhat UM, Acharya RV, Prabhu GKJ (2012) Image analysis approach for development of a decision support system for detection of malaria parasites in thin blood smear images. J Digit Imaging 25(4):542–549
Proakis JG, Manolakis DG (2001) Digital signal processing: principles algorithms and applications. Prentice-Hall, USA
Purwar Y, Shah SL, Clarke G, Almugairi A, Muehlenbachs A (2011) Automated and unsupervised detection of malaria parasites in microscopic images. Malar J 10:364
Rodríguez A, Guil N, Shotton D (2005) Analysis and description of the semantic content of cell biological videos. Multimed Tools Appl. 25(1):37–58
Roy A, Singha J, Devi SS, Laskar RH (2016) Impulse noise removal using SVM classification based fuzzy filter from gray scale images. Signal Process 128:262–273
Russell S, Norvig P (2003) Artificial Intelligence: A modern approach, 2nd edn. Prentice Hall, Upper Saddle River
Ruta D, Gabrys B (2005) Classifier selection for majority voting. Inform Fusion 6(1):63–81
Savkare S, Narote S (2011) Automatic detection of malaria parasites for estimating parasitemia. Int J Comput Sci Secur 5(3):310–315
Sertel O, Dogdas B, Chui CS, Gurcan MN (2011) Microscopic image analysis for quantitative characterization of muscle fiber type composition. Comput Med Imaging Graph 35(7–8):616–628
Siggelkow S (2002) Feature histograms for content-based image retrieval. Dissertation, Universitat Freiburg
Soni J, Mishra N, Kamargaonkar NC (2011) Automatic difference between RBC and malaria parasites based on morphology with first order features using image processing. Int J Adv Eng Tech 1(5):290–297
Springl V (2009) Automatic malaria diagnosis through microscopic imaging. Faculty of Electrical Engineering, Prague
Sun F, Xu Y, Zhou J (2016) Active learning SVM with regularization path for image classification. Multimed Tools Appl. 75(3):1427–1442
Tek FB, Dempster AG, Kale I (2006a) Malaria parasite detection in peripheral blood images. In: Proceeding of the British Machine Vision Conference, UK, pp 347–356
Tek FB, Dempster AG, Kale I (2006b) A colour normalization method for giemsa-stained blood cell images. In: Proceeding of Signal Processing and Communications Applications, IEEE, 2006
Tek FB, Dempster AG, Kale I (2010) Parasite detection and identification for automated thin blood film malaria diagnosis. Comput Vis Image Und 114(1):21–32
Terrillon JC, Shirazi MN, Fukamachi H, Akamatsu S (2000) Comparative performance of different skin chrominance models and chrominance spaces for the automatic detection of human faces in color images. In Proceedings of 4th IEEE Automatic Face and Gesture Recognition. 2000, pp 54–61
Theodoridis S, Pikrakis A, Koutroumbas K, Cavouras D (2010) Introduction to pattern recognition: a MATLAB approach, 4th edn. Academic Press, US, pp. 107–135
Weinberger KQ, Saul LK (2009) Distance metric learning for large margin nearest neighbor classification. J Mach Learn Res 10:207–244
WHO (2013) World malaria report
Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH (2007) A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). AmJTrop Med Hyg 77(6):119–127
Zhu W, Huang W, Lin Z (2016) Data and feature mixed ensemble based extreme learning machine for medical object detection and segmentation. Multimed Tools Appl 75(5):2815–2837
Acknowledgements
This work is supported by the Speech and Image Processing Lab under Department of ECE at National Institute of Technology, Silchar, India.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s11042-017-4490-7.
Rights and permissions
About this article
Cite this article
Devi, S.S., Roy, A., Singha, J. et al. Malaria infected erythrocyte classification based on a hybrid classifier using microscopic images of thin blood smear. Multimed Tools Appl 77, 631–660 (2018). https://doi.org/10.1007/s11042-016-4264-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11042-016-4264-7