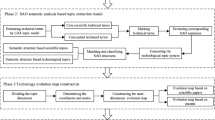
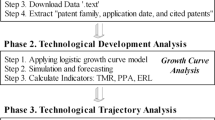
Abstract
Technological change evolves along a cyclical divergent-convergent pattern in knowledge diffusion paths. Technological divergence occurs as a breakthrough innovation, or discontinuity, inaugurating an era of ferment in which several competing technologies emerge and gradually advance. Technological convergence occurs as a series of evolutionary, variant changes that are gradually combined or fused together to open the industry to successive dominant designs or guideposts. To visualize such a pattern of technological evolution, we choose to study lithium iron phosphate (LFP) battery technology through an extension of the citation-based main path analysis, namely the key-route main path analysis. The key-route method discloses the main paths that travel through a specified number of key citations. The resulting multiple paths reveal the structure of the knowledge diffusion paths. The citation network is constructed from 1,531 academic articles on LFP battery technology published between 1997 and early 2012. Findings illustrate that LFP battery technology has completed two full technological cycles and is in the middle of the third cycle.




Similar content being viewed by others
References
Abernathy, W. J., & Utterback, J. M. (1975). A dynamic model of product and process innovation. Omega, 3, 639–656.
Adams, S. (2010). Lithium ion pathways in LiFePO(4) and related olivines. Journal of Solid State Electrochemistry, 14(10), 1787–1792.
Adams, S., & Rao, R. (2011). Simulated defect and interface engineering for high power Li electrode materials. Solid State Ionics, 184(1), 57–61.
Anderson, P., & Tushman, M. (1990). Technological discontinuities and dominant designs: a cyclical model of technological change. Administrative Science Quarterly, 35(4), 604–633.
Andersson, A., Kalska, B., Haggstrom, L., & Thomas, J. (2000a). Lithium extraction/insertion in LiFePO4: an X-ray diffraction and Mössbauer spectroscopy study. Solid State Ionics, 130, 41–52.
Andersson, A., Thomas, J., Kalska, B., & Haggstrom, L. (2000b). Thermal stability of LiFePO4-based cathodes. Electrochemical and Solid State Letters, 3(2), 66–68.
Arthur, W. B. (1989). Competing technologies, increasing returns, and lock-in by historical events. The Economic Journal, 99, 116–131.
Axmann, P., Stinner, C., Wohlfahrt-Mehrens, M., Mauger, A., Gendron, F., & Julien, C. (2009). Nonstoichiometric LiFePO(4): defects and related properties. Chemistry of Materials, 21(8), 1636–1644.
Barker, J., Saidi, M., & Swoyer, J. (2003). Lithium iron(II) phospho-olivines prepared by a novel carbothermal reduction method. Electrochemical and Solid State Letters, 6(3), A53–A55.
Batagelj, V. (2003). Efficient algorithms for citation network analysis. University of Ljubljana, Institute of Mathematics, Physics and Mechanics, Department of Theoretical Computer Science.
Bhupatiraju, S., Nomaler, Ö., Triulzi, G., & Verspagen, B. (2012). Knowledge flows–analyzing the core literature of innovation, entrepreneurship and science and technology studies. Research Policy, 41(7), 1205–1218.
Breschi, S., & Lissoni, F. (2001). Knowledge spillovers and local innovation systems: a critical survey. Industrial and Corporate Change, 10(4), 975–1005.
Chen, G., Song, X., & Richardson, T. (2007). Metastable solid-solution phases in the LiFePO4/FePO4 system. Journal of the Electrochemical Society, 154(7), A627–A632.
Chung, S., Bloking, J., & Chiang, Y. (2002). Electronically conductive phospho-olivines as lithium storage electrodes. Nature Materials, 1(2), 123–128.
D’Urso, P., & Massari, R. (2013). Fuzzy clustering of human activity patterns. Fuzzy Sets and Systems, 215, 29–54.
David, B., Fernando, J., & Itziar, C. (2011). Mapping the importance of the real world: the validity of connectivity analysis of patent citations networks. Research Policy, 40(3), 473–486.
Delacourt, C., Poizot, P., Levasseur, S., & Masquelier, C. (2006). Size effects on carbon-free LiFePO4 powders. Electrochemical and Solid State Letters, 9(7), A352–A355.
Delacourt, C., Poizot, P., Tarascon, J., & Masquelier, C. (2005). The existence of a temperature-driven solid solution in LixFePO4 for 0 <= x <= 1. Nature Materials, 4(3), 254–260.
Delacourt, C., Wurm, C., Reale, P., Morcrette, M., & Masquelier, C. (2004). Low temperature preparation of optimized phosphates for Li-battery applications. Solid State Ionics, 173, 113–118.
Dosi, G. (1982). Technological paradigms and technological trajectories: a suggested interpretation of the determinants and directions of technical change. Research Policy, 11(3), 147–162.
Ellis, B., Perry, L., Ryan, D., & Nazar, L. (2006). Small polaron hopping in LixFePO4 solid solutions: coupled lithium-ion and electron mobility. Journal of the American Chemical Society, 128(35), 11416–11422.
Fergus, J. (2010). Recent developments in cathode materials for lithium ion batteries. Journal of Power Sources, 195(4), 939–954.
Fisher, C., & Islam, M. (2008). Surface structures and crystal morphologies of LiFePO4: relevance to electrochemical behavior. Journal of Materials Chemistry, 18(11), 1209–1215.
Floyd, R. W. (1962). Algorithm 97: shortest path. Communications of the ACM, 5(6), 345.
Garfield, E. (1972). Citation analysis as a tool in journal evaluation. Science, 178, 471–479.
Gibot, P., Casas-Cabanas, M., Laffond, L., Levasseur, S., Carlach, P., Hamelet, S., et al. (2008). Room-temperature single-phase Li insertion/extraction in nanoscale LixFePO4. Nature Materials, 7, 741–747.
Hui, S. K., Fader, P. S., & Bradlow, E. T. (2009). Path data in marketing: an integrative framework and prospectus for model building. Marketing Science, 28(2), 320–335.
Hummon, N., & Doreian, P. (1989). Connectivity in a citation network: the development of DNA theory. Social Networks, 11(1), 39–63.
Hung, A. (2004). Explaining the process of innovation: the dynamic reconciliation of action and structure. Human Relations, 57(11), 1479–1497.
Kobayashi, G., Nishimura, S.I., Park, M.S., Kanno, R., Yashima, M., Ida, T., & Yamada, A. (2009) Isolation of solid solution phases in size-controlled Li(x)FePO(4) at room temperature. 19(3), 395–403.
Kodama, F. (1992). Technology fusion and the new R&D. Harvard Business Review, 70(4), 70–78.
Kruskal, J. B. (1983a). An overview of sequence comparison: time warps, string edits, and macromolecules. SIAM review, 25(2), 201–237.
Kruskal, J. B. (1983b). An overview of sequence comparison. In D. Sankoff & J. B. Kruskal (Eds.), Time warps, string edits, and macromolecules: the theory and practice of sequence comparison (pp. 1–44). Reading: Addison-Wesley Publishing Company.
Laffont, L., Delacourt, C., Gibot, P., Wu, M., Kooyman, P., Masquelier, C., et al. (2006). Study of the LiFePO4/FePO4 two-phase system by high-resolution electron energy loss spectroscopy. Chemistry of Materials, 18(23), 5520–5529.
Levinthal, D. A. (1998). The slow pace of rapid technological change: gradualism and punctuation in technological change. Industrial and Corporate Change, 7(2), 217–247.
Liu, J. S., & Lu, L. Y. Y. (2012). An integrated approach for the main path analysis: The development of the hirsch index as an example. Journal of the American Society for Information Science and Technology, 63(3), 528–542.
Lundvall, B.-Å. (Ed.). (1992). National systems of innovation: towards a theory of innovation and interactive learning. London: Pinter.
Martinelli, A. (2012). An emerging paradigm or just another trajectory? Understanding the nature of technological changes using engineering heuristics in the telecommunications switching industry. Research Policy, 41(2), 414–429.
Matsui, H., Nakamura, T., Kobayashi, Y., Tabuchi, M., & Yamada, Y. (2010). Open-circuit voltage study on LiFePO4 olivine cathode. Journal of Power Sources, 195(19), 6879–6883.
Maxisch, T., Zhou, F., & Ceder, G. (2006). Ab initio study of the migration of small polarons in olivine LixFePO4 and their association with lithium ions and vacancies. Physical Review B, 73(10), 104301.
Mina, A., Ramlogan, R., Tampubolon, G., & Metcalfe, J. (2007). Mapping evolutionary trajectories: applications to the growth and transformation of medical knowledge. Research Policy, 36(5), 789–806.
Nelson, R., & Winter, S. (1982). An evolution theory of economic change. Cambridge: Harvard University Press.
Padhi, A., Nanjundaswamy, K., & Goodenough, J. (1997a). Phospho-olivines as positive-electrode materials for rechargeable lithium batteries. Journal of the Electrochemical Society, 144(4), 1188–1194.
Padhi, A., Nanjundaswamy, K., Masquelier, C., Okada, S., & Goodenough, J. (1997b). Effect of structure on the Fe3+/Fe2+ redox couple in iron phosphates. Journal of the Electrochemical Society, 144(5), 1609–1613.
Pearson, W. R., & Lipman, D. J. (1988). Improved tools for biological sequence comparison. Proceedings of the National Academy of Sciences, 85(8), 2444–2448.
Porter, A., Youtie, J., Shapira, P., & Schoeneck, D. (2008). Refining search terms for nanotechnology. Journal of Nanoparticle Research, 10(5), 715–728.
Ramana, C., Mauger, A., Gendron, F., Julien, C., & Zaghib, K. (2009). Study of the Li-insertion/extraction process in LiFePO(4)/FePO(4). Journal of Power Sources, 187(2), 555–564.
Safari, M., & Delacourt, C. (2011a). Mathematical modeling of lithium iron phosphate electrode: galvanostatic charge/discharge and path dependence. Journal of the Electrochemical Society, 158(2), A63–A73.
Safari, M., & Delacourt, C. (2011b). Modeling of a commercial graphite/LiFePO4 cell. Journal of the Electrochemical Society, 158(5), A562–A571.
Safari, M., & Delacourt, C. (2011c). Aging of a commercial graphite/LiFePO4 cell. Journal of the Electrochemical Society, 158(10), A1123–A1135.
Sahal, D. (1985). Technological guideposts and innovation avenues. Research Policy, 14(2), 61–82.
Singh, J. (2005). Collaborative networks as determinants of knowledge diffusion patterns. Management Science, 51(5), 756–770.
Srinivasan, V., & Newman, J. (2006). Existence of path-dependence in the LiFePO4 electrode. Electrochemical and Solid State Letters, 9(3), A110–A114.
Tang, P., & Holzwarth, N. (2003). Electronic structure of FePO4, LiFePO4, and related materials. Physical Review B, 68(16), 165107.
Thomke, S., & Kuemmerle, W. (2002). Asset accumulation, interdependence and technological change: evidence from pharmaceutical drug discovery. Strategic Management Journal, 23(7), 619–635.
Tucker, M., Doeff, M., Richardson, T., Finones, R., Reimer, J., & Cairns, E. (2002). Li-7 and P-31 magic angle spinning nuclear magnetic resonance of LiFePO4-type materials. Electrochemical and Solid State Letters, 5(5), A95–A98.
Verspagen, B. (2007). Mapping technological trajectories as patent citation networks: a study on the history of fuel cell research. Advances in Complex Systems, 10(1), 93–115.
Wang, Y., He, P., & Zhou, H. (2011). Olivine LiFePO4: development and future. Energy and Environmental Science, 4(3), 805–817.
Yamada, A., & Chung, S. (2001). Crystal chemistry of the olivine-type Li(MnyFe1-y)PO4 and (MnyFe1-y)PO4 as possible 4 V cathode materials for lithium batteries. Journal of the Electrochemical Society, 148(8), A960–A967.
Yamada, A., Chung, S., & Hinokuma, K. (2001a). Optimized LiFePO4 for lithium battery cathodes. Journal of the Electrochemical Society, 148(3), A224–A229.
Yamada, A., Hosoya, M., Chung, S., Kudo, Y., Hinokuma, K., Liu, K., et al. (2003). Olivine-type cathodes achievements and problems. Journal of Power Sources, 119, 232–238.
Yamada, A., Koizumi, H., Nishimura, S., Sonoyama, N., Kanno, R., Yonemura, M., et al. (2006a). Room-temperature miscibility gap in Li(x)FePO(4). Nature Materials, 5(5), 357–360.
Yamada, A., Koizumi, H., Sonoyama, N., & Kanno, R. (2005a). Phase change in LixFePO4. Electrochemical and Solid State Letters, 8(8), A409–A413.
Yamada, A., Kudo, Y., & Liu, K. (2001b). Phase diagram of Li-x(MnyFe1-y)PO4 (0 <= x, y <= 1). Journal of the Electrochemical Society, 148(10), A1153–A1158.
Yamada, A., Takei, Y., Koizumi, H., Sonoyama, N., Kanno, R., Itoh, K., et al. (2006b). Electrochemical, magnetic, and structural investigation of the Li-x(MnyFe1-y)PO4 olivine phases. Chemistry of Materials, 18(3), 804–813.
Yamada, A., Yonemura, M., Takei, Y., Sonoyama, N., & Kanno, R. (2005b). Fast charging LiFePO4. Electrochemical and Solid State Letters, 8(1), A55–A58.
Yang, S., Song, Y., Zavalij, P., & Whittingham, M. (2002). Reactivity, stability and electrochemical behavior of lithium iron phosphates. Electrochemistry Communications, 4(3), 239–244.
Yang, S., Zavalij, P., & Whittingham, M. (2001). Hydrothermal synthesis of lithium iron phosphate cathodes. Electrochemistry Communications, 3(9), 505–508.
Yonemura, M., Yamada, A., Takei, Y., Sonoyama, N., & Kanno, R. (2004). Comparative kinetic study of olivine LixMPO4 (M = Fe, Mn). Journal of the Electrochemical Society, 151(9), A1352–A1356.
Yuan, L., Wang, Z., Zhang, W., Hu, X., Chen, J., Huang, Y., et al. (2011). Development and challenges of LiFePO4 cathode material for lithium-ion batteries. Energy and Environmental Science, 4(2), 269–284.
Zhang, W. (2011). Structure and performance of LiFePO4 cathode materials: a review. Journal of Power Sources, 196(6), 2962–2970.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hung, SC., Liu, J.S., Lu, L.Y.Y. et al. Technological change in lithium iron phosphate battery: the key-route main path analysis. Scientometrics 100, 97–120 (2014). https://doi.org/10.1007/s11192-014-1276-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11192-014-1276-9