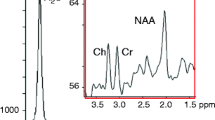
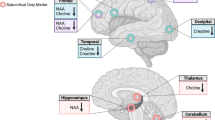
Abstract
Proton magnetic resonance spectroscopy (1H-MRS) now is widely used in clinical researches for the measurement of compounds or metabolites in vivo, especially in neuropsychiatric diseases/disorders. Recently, there are many studies on substance use disorders utilizing 1H-MRS to explore the mechanism of brain metabolites. It is found that metabolites levels in substance users are changed compared with healthy controls. Furthermore, these changes also relate to behavior indices, and provide evidence for the impact on neuronal health, energy metabolism and membrane turnover. However, 1H-MRS is not yet a mature detection technology, and it still has many challenges in the application of the neuropsychiatric disorder area. The settings of test parameters and the inconsistency of results across different studies still plague the clinicians and technicians. This article is intended to provide an overview of basic theory and methods of 1H-MRS, and the literature reporting metabolites alterations in substance dependence, as well as the related neuropsychological performance. At last, we will discuss the forthcoming challenges and possible future direction in this area.
Similar content being viewed by others
References
Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimag Clinics North Am, 2013, 23: 359–380
Karl A, Werner A. The use of proton magnetic resonance spectroscopy in PTSD research-meta-analyses of findings and methodological review. Neurosci Biobehavioral Rev, 2010, 34: 7–22
Lee R S C, Hoppenbrouwers S, Franken I. A systematic meta-review of impulsivity and compulsivity in addictive behaviors. Neuropsychol Rev, 2019, 29: 14–26
Zou Z, Wang H, d’Oleire Uquillas F, et al. Definition of substance and non-substance addiction. Adv Exp Med Biol, 2017, 1010: 21–41
Degenhardt L, Bharat C, Glantz M D, et al. The epidemiology of drug use disorders cross-nationally: findings from the WHO’s World Mental Health Surveys. Int J Drug Policy, 2019, 71: 103–112
United Nations Office on Drugs and Crime. World Drug Report 2018 by United Nations Office on Drugs and Crime. New York: United Nations, 2018. https://www.unodc.org/wdr2018/
Koob G F, Volkow N D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry, 2016, 3: 760–773
Hellem T, Shi X, Latendresse G, et al. The utility of magnetic resonance spectroscopy for understanding substance use disorders: a systematic review of the literature. J Am Psychiatr Nurses Assoc, 2015, 21: 244–275
Ernst R R, Bodenhausen G, Wokaun A, et al. Principles of Nuclear Magnetic Resonance in One and Two Dimensions. Oxford: Oxford University Press, 1987
Williams S. In Vivo NMR Spectroscopy: Principles and Techniques. Chichester: Wiley, 1998, 35: 201
Ende G. Proton magnetic resonance spectroscopy: relevance of glutamate and GABA to neuropsychology. Neuropsychol Rev, 2015, 25: 315–325
Bottomley P. 4480228 selective volume method for performing localized NMR spectroscopy. Magnetic Resonance Imag, 1985, 3: iv–v
Frahm J, Bruhn H, Gyngell M L, et al. Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med, 1989, 9: 79–93
Tamiya T, Kinoshita K, Ono Y, et al. Proton magnetic resonance spectroscopy reflects cellular proliferative activity in astrocytomas. Neuroradiology, 2000, 42: 333–338
Hammen T, Stefan H, Eberhardt K E, et al. Clinical applications of 1H-MR spectroscopy in the evaluation of epilepsies-what do pathological spectra stand for with regard to current results and what answers do they give to common clinical questions concerning the treatment of epilepsies? Acta Neurol Scand, 2003, 108: 223–238
Szulc A, Galińska B, Tarasów E, et al. N-acetylaspartate (NAA) levels in selected areas of the brain in patients with chronic schizophrenia treated with typical and atypical neuroleptics: a proton magnetic resonance spectroscopy (1H MRS) study. Medical Sci Monitor, 2007, 13: 17–22
Gruber S, Frey R, Mlynárik V, et al. Quantification of metabolic differences in the frontal brain of depressive patients and controls obtained by 1H-MRS at 3 Tesla. Invest Radiol, 2003, 38: 403–408
Frischknecht U, Hermann D, Tunc-Skarka N, et al. Negative association between MR-spectroscopic glutamate markers and gray matter volume after alcohol withdrawal in the hippocampus: a translational study in humans and rats. Alcohol Clin Exp Res, 2017, 41: 323–333
Burger A, Brooks S J, Stein D J, et al. The impact of acute and short-term methamphetamine abstinence on brain metabolites: a proton magnetic resonance spectroscopy chemical shift imaging study. Drug Alcohol Dependence, 2018, 185: 226–237
Yeo R A, Thoma R J, Gasparovic C, et al. Neurometabolite concentration and clinical features of chronic alcohol use: a proton magnetic resonance spectroscopy study. Psychiatry Res-Neuroimag, 2013, 211: 141–147
Crocker C E, Purdon S E, Hanstock C C, et al. Enduring changes in brain metabolites and executive functioning in abstinent cocaine users. Drug Alcohol Dependence, 2017, 178: 435–442
Howells F M, Uhlmann A, Temmingh H, et al. (1)H-magnetic resonance spectroscopy ((1)H-MRS) in methamphetamine dependence and methamphetamine induced psychosis. Schizophrenia Res, 2014, 153: 122–128
Licata S C, Renshaw P F. Neurochemistry of drug action: insights from proton magnetic resonance spectroscopic imaging and their relevance to addiction. Ann New York Acad Sci, 2010, 1187: 148–171
Hermann D, Weber-Fahr W, Sartorius A, et al. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol Psychiatry, 2012, 71: 1015–1021
Yang S, Salmeron B J, Ross T J, et al. Lower glutamate levels in rostral anterior cingulate of chronic cocaine users—a (1)H-MRS study using TE-averaged PRESS at 3 T with an optimized quantification strategy. Psychiatry Res, 2009, 174: 171–176
Prisciandaro J J, Tolliver B K, Prescot A P, et al. Unique prefrontal GABA and glutamate disturbances in co-occurring bipolar disorder and alcohol dependence. Transl Psychiatry, 2017, 7: e1163
Silveri M M, Cohen-Gilbert J, Crowley D J, et al. Altered anterior cingulate neurochemistry in emerging adult binge drinkers with a history of alcohol-induced blackouts. Alcohol Clin Exp Res, 2014, 38: 969–979
Bagga D, Khushu S, Modi S, et al. Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. J Stud Alcohol Drugs, 2014, 75: 817–826
Jiang L, Gulanski B I, de Feyter H M, et al. Increased brain uptake and oxidation of acetate in heavy drinkers. J Clin Invest, 2013, 123: 1605–1614
Ende G, Hermann D, Demirakca T, et al. Loss of control of alcohol use and severity of alcohol dependence in nontreatment-seeking heavy drinkers are related to lower glutamate in frontal white matter. Alcohol Clin Exp Res, 2013, 37: 1643–1649
Abé C, Mon A, Hoefer M E, et al. Metabolic abnormalities in lobar and subcortical brain regions of abstinent polysubstance users: magnetic resonance spectroscopic imaging. Alcohol Alcoholism, 2013, 48: 543–551
Abé C, Mon A, Durazzo T C, et al. Polysubstance and alcohol dependence: unique abnormalities of magnetic resonance-derived brain metabolite levels. Drug Alcohol Dependence, 2013, 130: 30–37
Xia Y, Ma D, Hu J, et al. Effect of metabotropic glutamate receptor 3 genotype on N-acetylaspartate levels and neurocognition in non-smoking, active alcoholics. Behav Brain Funct, 2012, 8: 42
Mon A, Durazzo T C, Meyerhoff D J. Glutamate, GABA, and other cortical metabolite concentrations during early abstinence from alcohol and their associations with neurocognitive changes. Drug Alcohol Dependence, 2012, 125: 27–36
Thoma R, Mullins P, Ruhl D, et al. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacol, 2011, 36: 1359–1365
Modi S, Bhattacharya M, Kumar P, et al. Brain metabolite changes in alcoholism: localized proton magnetic resonance spectroscopy study of the occipital lobe. Eur J Rad, 2011, 79: 96–100
Gazdzinski S, Durazzo T C, Mon A, et al. Cerebral white matter recovery in abstinent alcoholics-a multimodality magnetic resonance study. Brain, 2010, 133: 1043–1053
Schuff N, Neylan T C, Fox-Bosetti S, et al. Abnormal N-acetylaspartate in hippocampus and anterior cingulate in posttraumatic stress disorder. Psychiatry Res-Neuroimag, 2008, 162: 147–157
Gazdzinski S, Durazzo T C, Yeh P H, et al. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res-Neuroimag, 2008, 162: 133–145
Lee E, Jang D P, Kim J J, et al. Alteration of brain metabolites in young alcoholics without structural changes. Neuroreport, 2007, 18: 1511–1514
Ende G, Walter S, Welzel H, et al. Alcohol consumption significantly influences the MR signal of frontal cholinecontaining compounds. Neuroimage, 2006, 32: 740–746
Durazzo T C, Gazdzinski S, Banys P, et al. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcoholism-Clin Exp Res, 2004, 28: 1849–1860
Schweinsburg B C, Alhassoon O M, Taylor M J, et al. Effects of alcoholism and gender on brain metabolism. Am J Psychiatry, 2003, 160: 1180–1183
Parks M H, Dawant B M, Riddle W R, et al. Longitudinal brain metabolic characterization of chronic alcoholics with proton magnetic resonance spectroscopy. Alcoholism Clin Exp Res, 2002, 26: 1368–1380
Schweinsburg B C, Taylor M J, Alhassoon O M, et al. Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcoholism Clin Exp Res, 2001, 25: 924–934
Estilaei M R, Matson G B, Payne G S, et al. Effects of chronic alcohol consumption on the broad phospholipid signal in human brain: an in vivo 31P MRS study. Alcoholism Clin Exp Res, 2001, 25: 89–97
Estilaei M R, Matson G B, Payne G S, et al. Effects of abstinence from alcohol on the broad phospholipid signal in human brain: an in vivo 31P magnetic resonance spectroscopy study. Alcoholism Clin Exp Res, 2001, 25: 1213–1220
Bendszus M, Weijers H G, Wiesbeck G, et al. Sequential MR imaging and proton MR spectroscopy in patients who underwent recent detoxification for chronic alcoholism: correlation with clinical and neuropsychological data. Am J Neuroradiol, 2001, 22: 1926–1932
Schweinsburg B C, Taylor M J, Videen J S, et al. Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: a preliminary MR spectroscopy study. Alcoholism Clin Exp Res, 2000, 24: 699–705
Seitz D, Widmann U, Seeger U, et al. Localized proton magnetic resonance spectroscopy of the cerebellum in detoxifying alcoholics. Alcoholism Clin Exp Res, 1999, 23: 158–163
Jagannathan N R, Desai N G, Raghunathan P. Brain metabolite changes in alcoholism: An in vivo proton magnetic resonance spectroscopy (MRS) study. Magn Reson Imag, 1996, 14: 553–557
Meyerhoff D J, MacKay S, Sappey-Marinier D, et al. Effects of chronic alcohol abuse and HIV infection on brain phosphorus metabolites. Alcoholism Clin Exp Res, 1995, 19: 685–692
Martin P R, Gibbs S J, Nimmerrichter A A, et al. Brain proton magnetic resonance spectroscopy studies in recently abstinent alcoholics. Alcoholism Clin Exp Res, 1995, 19: 1078–1082
Bartsch A J, Homola G, Biller A, et al. Manifestations of early brain recovery associated with abstinence from alcoholism. Brain, 2007, 130: 36–47
Bauer J, Pedersen A, Scherbaum N, et al. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacol, 2013, 38: 1401–1408
Ende G, Welzel H, Walter S, et al. Monitoring the effects of chronic alcohol consumption and abstinence on brain metabolism: a longitudinal proton magnetic resonance spectroscopy study. Biol Psychiatry, 2005, 58: 974–980
Frye M A, Hinton D J, Karpyak V M, et al. Anterior cingulate glutamate is reduced by acamprosate treatment in patients with alcohol dependence. J Clin PsychoPharmacol, 2016, 36: 669–674
Schulte M, Goudriaan A E, Kaag A M, et al. The effect of N-acetylcysteine on brain glutamate and gamma-aminobutyric acid concentrations and on smoking cessation: a randomized, double-blind, placebo-controlled trial. J Psychopharmacol, 2017, 31: 1377–1379
Schulte M H J, Kaag A M, Wiers R W, et al. Prefrontal Glx and GABA concentrations and impulsivity in cigarette smokers and smoking polysubstance users. Drug Alcohol Dependence, 2017, 179: 117–123
Gallinat J, Schubert F. Regional cerebral glutamate concentrations and chronic tobacco consumption. Pharmacopsychiatry, 2007, 40: 64–67
Mennecke A, Gossler A, Hammen T, et al. Physiological effects of cigarette smoking in the limbic system revealed by 3 tesla magnetic resonance spectroscopy. J Neural Transm, 2014, 121: 1211–1219
O’Neill J, Tobias M C, Hudkins M, et al. Glutamatergic neurometabolites during early abstinence from chronic methamphetamine abuse. Int J Neuropsychopharmacol, 2014, 18: pyu059
Crocker C E, Bernier D C, Hanstock C C, et al. Prefrontal glutamate levels differentiate early phase schizophrenia and methamphetamine addiction: a (1)H MRS study at 3Tesla. Schizophrenia Res, 2014, 157: 231–237
Sung Y H, Yurgelun-Todd D A, Shi X F, et al. Decreased frontal lobe phosphocreatine levels in methamphetamine users. Drug Alcohol Dependence, 2013, 129: 102–109
Sung Y H, Carey P D, Stein D J, et al. Decreased frontal N-acetylaspartate levels in adolescents concurrently using both methamphetamine and marijuana. Behavioural Brain Res, 2013, 246: 154–161
Cloak C C, Alicata D, Chang L, et al. Age and sex effects levels of choline compounds in the anterior cingulate cortex of adolescent methamphetamine users. Drug Alcohol Dependence, 2011, 119: 207–215
Sung Y H, Cho S C, Hwang J, et al. Relationship between N-acetyl-aspartate in gray and white matter of abstinent methamphetamine abusers and their history of drug abuse: a proton magnetic resonance spectroscopy study. Drug Alcohol Dependence, 2007, 88: 28–35
Chang L, Ernst T, Grob C S, et al. Cerebral1H MRS alterations in recreational 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) users. J Magn Reson Imag, 1999, 10: 521–526
Chang L, Ernst T, Speck O, et al. Additive effects of HIV and chronic methamphetamine use on brain metabolite abnormalities. Am J Psychiatry, 2005, 162: 361–369
Daumann J, Fischermann T, Pilatus U, et al. Proton magnetic resonance spectroscopy in ecstasy (MDMA) users. Neurosci Lett, 2004, 362: 113–116
Ernst T, Chang L, Leonido-Yee M, et al. Evidence for long-term neurotoxicity associated with methamphetamine abuse: a 1H MRS study. Neurology, 2000, 54: 1344–1349
Nordahl T E, Salo R, Natsuaki Y, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry, 2005, 62: 444–452
Nordahl T E, Salo R, Possin K, et al. Low N-acetyl-aspartate and high choline in the anterior cingulum of recently abstinent methamphetamine-dependent subjects: a preliminary proton MRS study. Psychiatry Res-Neuroimag, 2002, 116: 43–52
Reneman L, Majoie C B, Flick H, et al. Reduced N-acetylaspartate levels in the frontal cortex of 3,4-methylenedioxymethamphetamine (Ecstasy) users: preliminary results. Am J Neuroradiol, 2002, 23: 231–237
Reneman L, Majoie C B L M, Schmand B, et al. Prefrontal N-acetylaspartate is strongly associated with memory performance in (abstinent) ecstasy users: preliminary report. Biol Psychiatry, 2001, 50: 550–554
Ernst T, Chang L. Adaptation of brain glutamate plus glutamine during abstinence from chronic methamphetamine use. J Neuroimmune Pharmacol, 2008, 3: 165–172
Lin J C, Jan R K, Kydd R R, et al. Investigating the microstructural and neurochemical environment within the basal ganglia of current methamphetamine abusers. Drug Alcohol Dependence, 2015, 149: 122–127
Salo R, Buonocore M H, Leamon M, et al. Extended findings of brain metabolite normalization in MA-dependent subjects across sustained abstinence: a proton MRS study. Drug Alcohol Dependence, 2011, 113: 133–138
Salo R, Nordahl T E, Buonocore M H, et al. Spatial inhibition and the visual cortex: a magnetic resonance spectroscopy imaging study. Neuropsychologia, 2011, 49: 830–838
Salo R, Nordahl T E, Natsuaki Y, et al. Attentional control and brain metabolite levels in methamphetamine abusers. Biol Psychiatry, 2007, 61: 1272–1280
Yang W, Yang R, Luo J, et al. Increased absolute glutamate concentrations and glutamate-to-creatine ratios in patients with methamphetamine use disorders. Front Psychiatry, 2018, 9: 368
Liu X L, Li L, Li J N, et al. Quantifying absolute glutamate concentrations in nucleus accumbens of prescription opioid addicts by using (1)H MRS. Brain Behav, 2017, 7: e00769
Greenwald M K, Woodcock E A, Khatib D, et al. Methadone maintenance dose modulates anterior cingulate glutamate levels in heroin-dependent individuals: a preliminary in vivo (1)H MRS study. Psychiatry Res-Neuroimag, 2015, 233: 218–224
Hermann D, Frischknecht U, Heinrich M, et al. MR spectroscopy in opiate maintenance therapy: association of glutamate with the number of previous withdrawals in the anterior cingulate cortex. Addiction Biol, 2012, 17: 659–667
Murray D E, Durazzo T C, Schmidt T P, et al. Frontal metabolite concentration deficits in opiate dependence relate to substance use, cognition, and self-regulation. J Addiction Res Therapy, 2016, 7: 286
Silveri M M, Pollack M H, Diaz C I, et al. Cerebral phosphorus metabolite and transverse relaxation time abnormalities in heroin-dependent subjects at onset of methadone maintenance treatment. Psychiatry Res-Neuroimag, 2004, 131: 217–226
Verdejo-García A, Lubman D I, Roffel K, et al. Cingulate biochemistry in heroin users on substitution pharmacotherapy. Aust New Zealand J Psychiatry, 2013, 47: 244–249
Yücel M, Lubman D I, Harrison B J, et al. Neuronal, physiological and brain-behavioural abnormalities in opiate-addicted individuals. Mol Psychiatry, 2007, 12: 611
Bitter S M, Weber W A, Chu W J, et al. N-acetyl aspartate levels in adolescents with bipolar and/or cannabis use disorders. J Dual Diagnosis, 2014, 10: 39–43
van de Giessen E, Weinstein J J, Cassidy C M, et al. Deficits in striatal dopamine release in cannabis dependence. Mol Psychiatry, 2017, 22: 68–75
Hermann D, Sartorius A, Welzel H, et al. Dorsolateral prefrontal cortex N-acetylaspartate/total creatine (NAA/tCr) loss in male recreational cannabis users. Biol Psychiatry, 2007, 61: 1281–1289
Silveri M M, Jensen J E, Rosso I M, et al. Preliminary evidence for white matter metabolite differences in marijuana-dependent young men using 2D J-resolved magnetic resonance spectroscopic imaging at 4 Tesla. Psychiatry Res-Neuroimag, 2011, 191: 201–211
Mashhoon Y, Jensen J E, Sneider J T, et al. Lower left thalamic Myo-inositol levels associated with greater cognitive impulsivity in marijuana-dependent young men: preliminary spectroscopic evidence at 4T. J Add Res Therapy, 2013, 4: 009
Prescot A P, Locatelli A E, Renshaw P F, et al. Neurochemical alterations in adolescent chronic marijuana smokers: a proton MRS study. Neuroimage, 2011, 57: 69–75
Muetzel R L, Marjańska M, Collins P F, et al. In vivo (1)H magnetic resonance spectroscopy in young-adult daily marijuana users. Neuroimage-Clin, 2013, 2: 581–589
Prescot A P, Renshaw P F, Yurgelun-Todd D A. γ-Amino butyric acid and glutamate abnormalities in adolescent chronic marijuana smokers. Drug Alcohol Dependence, 2013, 129: 232–239
Chang L, Ernst T, Strickland T, et al. Gender effects on persistent cerebral metabolite changes in the frontal lobes of abstinent cocaine users. Am J Psychiatry, 1999, 156: 716–722
Martinez D, Slifstein M, Nabulsi N, et al. Imaging glutamate homeostasis in cocaine addiction with the metabotropic glutamate receptor 5 positron emission tomography radiotracer [(11)C]ABP688 and magnetic resonance spectroscopy. Biol Psychiatry, 2014, 75: 165–171
Li S J, Wang Y, Pankiewicz J, et al. Neurochemical adaptation to cocaine abuse: reduction of N-acetyl aspartate in thalamus of human cocaine abusers. Biol Psychiatry, 1999, 45: 1481–1487
Hulka L M, Scheidegger M, Vonmoos M, et al. Glutamatergic and neurometabolic alterations in chronic cocaine users measured with (1) H-magnetic resonance spectroscopy. Addiction Biol, 2016, 21: 205–217
Ke Y, Streeter C C, Nassar L E, et al. Frontal lobe GABA levels in cocaine dependence: a two-dimensional, J-resolved magnetic resonance spectroscopy study. Psychiatry Res-Neuroimag, 2004, 130: 283–293
Schmaal L, Veltman D J, Nederveen A, et al. N-acetylcysteine normalizes glutamate levels in cocaine-dependent patients: a randomized crossover magnetic resonance spectroscopy study. Neuropsychopharmacol, 2012, 37: 2143–2152
Durazzo T C, Gazdzinski S, Banys P, et al. Cigarette smoking exacerbates chronic alcohol-induced brain damage: a preliminary metabolite imaging study. Alcohol Clinical Exp Res, 2005, 28: 1849–1860
Meyerhoff D J, Blumenfeld R, Truran D, et al. Effects of heavy drinking, binge drinking, and family history of alcoholism on regional brain metabolites. Alcohol Clin Exp Res, 2004, 28: 650–661
Mason G F, Krystal J H. MR spectroscopy: its potential role for drug development for the treatment of psychiatric diseases. NMR Biomed, 2006, 19: 690–701
Prisciandaro J J, Schacht J P, Prescot A P, et al. Associations between recent heavy drinking and dorsal anterior cingulate N-acetylaspartate and glutamate concentrations in non-treatment-seeking individuals with alcohol dependence. Alcohol Clin Exp Res, 2016, 40: 491–496
Meyerhoff D J, Murray D E, Durazzo T C, et al. Brain GABA and glutamate concentrations following chronic gabapentin administration: a convenience sample studied during early abstinence from alcohol. Front Psychiatry, 2018, 9: 78
Forstera B, Castro P A, Moraga-Cid G, et al. Potentiation of Gamma aminobutyric acid receptors (GABAAR) by ethanol: how are inhibitory receptors affected? Front Cell Neurosci, 2016, 10: 114
Stephens D N, King S L, Lambert J J, et al. GABAA receptor subtype involvement in addictive behaviour. Genes Brain Behav, 2017, 16: 149–184
Alasmari F, Goodwani S, McCullumsmith R E, et al. Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence. Prog Neurobiol, 2018, 171: 32–49
Gallinat J, Lang U E, Jacobsen L K, et al. Abnormal hippocampal neurochemistry in smokers: evidence from proton magnetic resonance spectroscopy at 3T. J Clin Psychopharmacol, 2007, 27: 80–84
Mashhoon Y, Janes A C, Jensen J E, et al. Anterior cingulate proton spectroscopy glutamate levels differ as a function of smoking cessation outcome. Progress Neuro-Psychopharmacol Biol Psychiatry, 2011, 35: 1709–1713
Nordahl T E, Salo R, Natsuaki Y, et al. Methamphetamine users in sustained abstinence: a proton magnetic resonance spectroscopy study. Arch General Psychiatry, 2005, 62: 444
Liu H S, Chou M C, Chung H W, et al. Potential long-term effects of MDMA on the basal ganglia-thalamocortical circuit: a proton MR spectroscopy and diffusion-tensor imaging study. Radiology, 2011, 260: 531–540
Chang L, Mehringer C M, Ernst T, et al. Neurochemical alterations in asymptomatic abstinent cocaine users: a proton magnetic resonance spectroscopy study. Biol Psychiatry, 1997, 42: 1105–1114
Thompson P M, Andreassen O A, Arias-Vasquez A, et al. ENIGMA and the individual: predicting factors that affect the brain in 35 countries worldwide. Neuroimage, 2017, 145: 389–408
Morley K C, Lagopoulos J, Logge W, et al. Neurometabolite levels in alcohol use disorder patients during baclofen treatment and prediction of relapse to heavy drinking. Front Psychiatry, 2018, 9: 412
Frye M A, Hinton D J, Karpyak V M, et al. Elevated glutamate levels in the left dorsolateral prefrontal cortex are associated with higher cravings for alcohol. Alcohol Clin Exp Res, 2016, 40: 1609–1616
Cowan R L, Joers J M, Dietrich M S. N-acetylaspartate (NAA) correlates inversely with cannabis use in a frontal language processing region of neocortex in MDMA (Ecstasy) polydrug users: a 3T magnetic resonance spectroscopy study. Pharmacol Biochem Behav, 2009, 92: 105–110
Wu Q, Qi C, Long J, et al. Metabolites alterations in the medial prefrontal cortex of methamphetamine users in abstinence: a (1)H MRS study. Front Psychiatry, 2018, 9: 478
Xing X X, Zuo X N. The anatomy of reliability: a must read for future human brain mapping. Sci Bull, 2018, 63: 1606–1607
Zuo X N, Xu T, Milham M P. Harnessing reliability for neuroscience research. Nat Hum Behav, 2019, 3: 768–771
Baeshen A, Wyss P O, Henning A, et al. Test-retest reliability of the brain metabolites GABA and Glx with JPRESS, PRESS, and MEGA-PRESS MRS sequences in vivo at 3T. J Magn Reson Imag, 2019, 51: 1181–1191
Shungu D C, Mao X, Gonzales R, et al. Brain γ-aminobutyric acid (GABA) detection in vivo with the J-editing (1)H MRS technique: a comprehensive methodological evaluation of sensitivity enhancement, macromolecule contamination and test-retest reliability. NMR Biomed, 2016, 29: 932–942
Prisciandaro J J, Mikkelsen M, Saleh M G, et al. An evaluation of the reproducibility of (1)H-MRS GABA and GSH levels acquired in healthy volunteers with J-difference editing sequences at varying echo times. Magn Reson Imag, 2020, 65: 109–113
Jensen J E, Auerbach R P, Pisoni A, et al. Localized MRS reliability of in vivo glutamate at 3T in shortened scan times: a feasibility study. NMR Biomed, 2017, 30: e3771
Morris P, Bachelard H. Reflections on the application of 13C-MRS to research on brain metabolism. NMR Biomed, 2003, 16: 303–312
Lanz B, Xin L, Millet P, et al. In vivo quantification of neuro-glial metabolism and glial glutamate concentration using 1H-[13C] MRS at 14.1T. J Neurochem, 2014, 128: 125–139
Sibson N R, Dhankhar A, Mason G F, et al. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA, 1998, 95: 316–321
Illes P, Burnstock G, Tang Y. Astroglia-derived ATP modulates CNS neuronal circuits. Trends Neurosci, 2019, 42: 885–898
Voineskos D, Blumberger D M, Zomorrodi R, et al. Altered transcranial magnetic stimulation-electroencephalographic markers of inhibition and excitation in the dorsolateral prefrontal cortex in major depressive disorder. Biol Psychiatry, 2019, 85: 477–486
Shine J M, Poldrack R A. Principles of dynamic network reconfiguration across diverse brain states. Neuroimage, 2018, 180: 396–405
Khanna A, Pascual-Leone A, Michel C M, et al. Microstates in resting-state EEG: current status and future directions. Neurosci Biobehaval Rev, 2015, 49: 105–113
Acknowledgements
This work was supported by National Key Research and Development Program of China (Grant No. 2017YFC1310400), National Nature Science Foundation (Grant No. 81771436, 81801319), Shanghai Municipal Health and Family Planning Commission (Grant No. 2017ZZ02021), Shanghai Key Laboratory of Psychotic Disorders (Grant No. 13DZ2260500), Program of Shanghai Academic Research Leader (Grant No. 17XD1403300), and Shanghai Municipal Science and Technology Major Project (Grant No. 2018SHZDZX05), and Shanghai Clinical Research Center for Mental Health (Grant No. 19MC1911100).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The author(s) declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chen, T., Tan, H., Lei, H. et al. Proton magnetic resonance spectroscopy in substance use disorder: recent advances and future clinical applications. Sci. China Inf. Sci. 63, 170101 (2020). https://doi.org/10.1007/s11432-019-2818-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11432-019-2818-5