Abstract
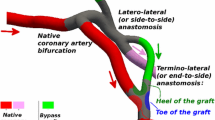
In coronary artery bypass graft (CABG) surgery the involved tissues are overstretched, which may lead to intimal hyperplasia and graft failure. We propose a computational methodology for the simulation of traditional CABG surgery, and analyze the effect of two clinically relevant parameters on the artery and graft responses, i.e., incision length and insertion angle for a given graft diameter. The computational structural analyses are based on actual three-dimensional vessel dimensions of a human coronary artery and a human saphenous vein. The analyses consider the structure of the end-to-side anastomosis, the residual stresses and the typical anisotropic and nonlinear vessel behaviors. The coronary artery is modeled as a three-layer thick-walled tube. The finite element method is employed to predict deformation and stress distribution at various stages of CABG surgery. Small variations of the arterial incision have relatively big effects on the size of the arterial opening, which depends solely on the residual stress state. The incision length has a critical influence on the graft shape and the stress in the graft wall. Stresses at the heel region are higher than those at the toe region. The changes in the mechanical environment are severe along all transitions between the venous tissue and the host artery. Particular stress concentrations occur at the incision ends. The proposed computational methodology may be useful in designing a coronary anastomotic device for reducing surgical trauma. It may improve the quantitative knowledge of vessel diseases and serve as a tool for virtual planning of vascular surgery.








Similar content being viewed by others
References
Ahmed SR, Johansson BL, Karlsson MB, Souza DS, Dashwood MR, Loesch A (2004) Human saphenous vein and coronary bypass surgery: ultrastructural aspects of conventional and ‘no-touch’ vein graft preparations. Histol Histopathol 19:421–433
Ballyk PD, Walsh C, Butany J, Ojha M (1998) Compliance mismatch may promote graft artery intimal hyperplasia by altering suture-line stresses. J Biomech 31:229–237
Bassiouny HS, White S, Glagov S, Choi E, Giddens DP, Zarins CK (1992) Anastomotic intimal hyperplasia: mechanical injury or flow induced. J Vasc Surg 15:708–716
Chamiot-Clerc P, Copie X, Renaud JF, Safar M, Girerd X (1998) Comparative reactivity and mechanical properties of human isolated internal mammary and radial arteries. Cardiovasc Res 37:811–819
Donovan DL, Schmidt SP, Townsend SP, Njus GO, Sharp WV (1990) Material and structural characterization of human saphenous vein. J Vasc Surg 12:531–537
Fung YC, Liu SQ (1989) Change of residual strains in arteries due to hypertrophy caused by aortic constrictions. Circ Res 65:1340–1349
Gu H, Chua A, Tan BK, Chew Hung K (2006) Nonlinear finite element simulation to elucidate the efficacy of slit arteriotomy for end-to-side arterial anastomosis in microsurgery. J Biomech 39:435–443
Hofer M, Rappitsch G, Perktold K, Trubel W, Schima H (1996) Numerical study of wall mechanics and fluid dynamics in end-to-side anastomoses and correlation to intimal hyperplasia. J Biomech 29:1297–1308
Holzapfel GA, Gasser TC, Ogden RW (2000) A new constitutive framework for arterial wall mechanics and a comparative study of material models. J Elast 61:1–48
Holzapfel GA, Sommer G, Gasser CT, Regitnig P (2005) Determination of the layer-specific mechanical properties of human coronary arteries with non-atherosclerotic intimal thickening, and related constitutive modelling. Am J Physiol Heart Circ Physiol 289:H2048–H2058
Holzapfel GA, Stadler M, Gasser TC (2005) Changes in the mechanical environment of stenotic arteries during interaction with stents: computational assessment of parametric stent design. J Biomech Eng 127:166–180
Hughes PE, How TV (1996) Effects of geometry and flow division on flow structures in models of the distal end-to-side anastomosis. J Biomech 29:855–872
Humphrey JD (2002) Cardiovascular solid mechanics. Cells, tissues, and organs. Springer, New York
Kaimovitz B, Lanir Y, Kassab GS (2005) Large-scale 3-D geometric reconstruction of the porcine coronary arterial vasculature based on detailed anatomical data. Ann Biomed Eng 33:1517–1535
Leuprecht A, Perktold K, Prosi M, Berk T, Trubel W, Schima H (2002) Numerical study of hemodynamics and wall mechanics in distal end-to-side anastomoses of bypass grafts. J Biomech 35:225–236
Matsumoto T, Hayashi K (1994) Mechanical and dimensional adaptation of rat aorta to hypertension. J Biomech Eng 116:278–283
Meng X, Mavromatis K, Galis ZS (1999) Mechanical stretching of human saphenous vein grafts induces expression and activation of matrix-degrading enzymes associated with vascular tissue injury and repair. Exp Mol Pathol 66:227–237
Migliavacca F, Dubini G (2005) Computational modeling of vascular anastomoses. Biomech Model Mechanobio 3:235–250
Milesi V, Rebolledo A, Ayala-Paredes F, Sanz N, Tommasi J, Rinaldi GJ, et al (1998) Mechanical properties of human saphenous veins from normotensive and hypertensive patients. Ann Thorac Surg 66:455–461
Scheltes JS, van Andel CJ, Pistecky PV, Borst C (2003) Coronary anastomotic devices: blood-exposed non-intimal surface and coronary wall stress. J Thorac Cardiovasc Surg 126:191–199
Sottiurai VS, Yao JS, Batson RC, Sue SL, Jones R, Nakamura YA (1989) Distal anastomotic intimal hyperplasia: histopathologic character and biogenesis. Ann Vasc Surg 3:26–3
Stooker W, Gok M, Sipkema P, Niessen HW, Baidoshvili A, Westerhof N et al (2003) Pressure–diameter relationship in the human greater saphenous vein. Ann Thorac Surg 76:1533–1538
Taylor RL (2002) FEAP—a finite element analysis program, version 7.4 user manual. University of California at Berkeley, Berkeley
Townsend SP (1993) The material and structural characterization of human saphenous vein patch grafts. Master Thesis, University of Akron
Trubel W, Moritz A, Schima H, Raderer F, Scherer R, Ullrich R, et al (1992) Compliance and formation of distal anastomotic intimal hyperplasia in Dacron mesh tube constricted veins used as arterial bypass grafts. ASAIO J 40:M273–M278
Trubel W, Schima H, Czerny M, Perktold K, Schimeck MG, Polterauer P (2004) Experimental comparison of four methods of end-to-side anastomosis with expanded polytetrafluoroethylene. Br J Surg 91:159–167
Vaishnav RN, Vossoughi J (1983) Estimation of residual strains in aortic segments. In: Hall CW (ed) Biomedical engineering ii: recent developments. Pergamon Press, New York, pp 330–333
Acknowledgments
This research was financed by the Spanish Ministry of Science and Education under Project No. CICYT DPI2004-07410-C03 and Grant/Contract No. FP2000-5317 for FC; and by the DISHEART European Project (CRAFT-FP6-2002-SME-1).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cacho, F., Doblaré, M. & Holzapfel, G.A. A procedure to simulate coronary artery bypass graft surgery. Med Bio Eng Comput 45, 819–827 (2007). https://doi.org/10.1007/s11517-007-0201-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-007-0201-2