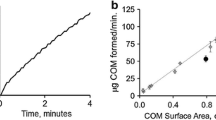
Abstract
Crystallization of calcium oxalate monohydrate in a section of a single kidney nephron (distal convoluted tubule) is simulated using a model adapted from industrial crystallization. The nephron fluid dynamics is represented as a crystallizer/separator series with changing volume to allow for water removal along the tubule. The model integrates crystallization kinetics and crystal size distribution and allows the prediction of the calcium oxalate concentration profile and the nucleation and growth rates. The critical supersaturation ratio for the nucleation of calcium oxalate crystals has been estimated as 2 and the mean crystal size as 1 μm. The crystal growth order, determined as 2.2, indicates a surface integration mechanism of crystal growth and crystal growth dispersion. The model allows the exploration of the effect of varying the input calcium oxalate concentration and the rate of water extraction, simulating real life stressors for stone formation such as dietary loading and dehydration.






Similar content being viewed by others
Abbreviations
- COM:
-
Calcium oxalate monohydrate
- CSD:
-
Crystal size distribution
- MSMPR:
-
Mixed suspension, mixed product removal
- PB:
-
Population balance
- WR:
-
Fractional water removal rate
References
Andreassen JP, Hounslow MJ (2004) Growth and aggregation of vaterite in seeded-batch experiments. AIChE J 50:2772–2782
Borissova A (2009) General systems modeling of multi-phase batch crystallization from solution. Chem Eng Process Process Intensif 48:268–278
Bramley AS, Hounslow MJ, Ryall RL (1997) Aggregation during precipitation from solution. Kinetics for calcium oxalate monohydrate. Chem Eng Sci 52:747–757
Brunsteiner M, Jones AG, Pratola F, Price SL, Simons SJR (2005) Toward a molecular understanding of crystal agglomeration. Cryst Growth Des 5:3–16
Dean JA (ed) (1979) Lange’s handbook of chemistry, 12th edn. McGraw-Hill, New York, p 4–35
Fasano JM, Khan RK (2001) Intratubular crystallization of calcium oxalate in the presence of membrane vesicles: an in vitro study. Kidney Int 59:169–178
Finlayson B (1972) The concept of a continuous crystallizer. Its theory and application to in vivo and in vitro urinary tract models. Investig Urol 9:258–263
Finlayson B, Reid F (1978) Expectation of free and fixed particles in urinary stone disease. Investig Urol 15:442–448
Garside J, Mersmann A, Nyvlt J (1990) Measurement of crystal growth rates. European Federation of Chemical Engineering, Working Party on Crystallization, Munich, p 37
Hartel RW, Randolph AD (1986) Mechanisms and kinetic modeling of calcium oxalate crystal aggregation in a urinelike liquor, part II: kinetic modeling. AIChE J 32:1186–1195
Hartel RW, Gottung BE, Randolph AD, Drach GW (1986) Mechanisms and kinetic modeling of calcium oxalate crystal aggregation in a urinelike liquor, part I: mechanisms. AIChE J 32:1176–1185
Hess B, Meinhardt U, Zipperle L, Giovanoli R (1995) Simultaneous measurements of calcium oxalate nucleation and aggregation: impact of various modifiers. Urol Res 23:231–238
Højgaard I, Tiselius HG (1999) Crystallization in the nephron. Urol Res 27:397–403
Højgaard I, Fornander A-M, Nilsson M-A, Tiselius HG (1999) The effect of pH changes on crystallization of calcium salts in solutions with an ion-composition corresponding to that in the distal tubule. Scanning Microsc 13:235–245
Hounslow MJ, Mumtaz HS, Collier AP, Barrick JP, Bramley AS (2001) A micro-mechanical model for the rate of aggregation during precipitation from solution. Chem Eng Sci 56:2543–2552
Kafarov VV, Dorohov IN, Koltzova E (1983) Systems analysis of chemical technology processes. Processes of mass crystallization from solutions and gas phase. Nauka, Moscow
Kavanagh JP (1992) Methods for the study of calcium oxalate crystallization and their application to urolithiasis research. Scanning Microsc 6:685–705
Kavanagh JP (1999) Enlargement of a lower pole calcium oxalate stone: a theoretical examination of the role of crystal nucleation, growth and aggregation. J Endourol 13:605–610
Kavanagh JP, Rao PN (2007) Lessons from a stone farm. AIP Conf Proc 900:159–169
Kelman RB (1965) Longitudinal diffusion along the nephron during stop flow. Bull Math Biol 27:53–56
Kevrekidis PG, Whitaker N (2003) Effect of backleak in nephron dynamics. Phys Rev E 67:061911
Kok DJ, Khan SR (1994) Calcium oxalate nephrolithiasis, a free or fixed particle disease. Kidney Int 46:847–854
Königsberger E, Königsberger L-C (2001) Thermodynamic modelling of crystal deposition in humans. Pure Appl Chem 73:785–797
Lide RD (ed) (1991). Handbook of chemistry & physics, 72nd edn. CRC Press, Boca Raton; Ann Arbor, Boston, p 4–49
Lieske JC, Leonard R, Toback FG (1995) Adhesion of calcium oxalate monohydrate crystals to renal epithelial cells is inhibited by specific anions. Am J Physiol Renal Physiol 268:F604–F612
May PM, Murray K (1991) JESS—a joint expert speciation system—I: raison d’être. Talanta 38:1409–1417
May PM, Murray K (1991) JESS—a joint expert speciation system—II: the thermodynamic database. Talanta 38:1419–1426
Mohan R, Myerson AS (2002) Growth kinetics: a thermodynamic approach. Chem Eng Sci 57:4277–4285
Mullin JW (2001) Crystallization, 4th edn. Butterworth-Heinemann, Oxford
Petrova EV, Gvozdev NV, Rashkovich LN (2004) Growth and dissolution of calcium oxalate monohydrate (COM) crystals. J Optoelectron Adv Mater 6:261–268
Randolph AD, Larson MA (1971) Theory of particulate processes. Academic Press, New York/London
Rodgers AL, Allie-Hamdulay S, Jackson G (2006) Therapeutic action of citrate in urolithiasis by chemical speciation: increase in pH is the determinant factor. Nephrol Dial Transplant 21:361–369
Schepers MSJ, Duim RAJ, Asselman M, Romijn JC, Schroder FH, Verkoelen CF (2003) Internalization of calcium oxalate crystals by renal tubular cells: a nephron segment-specific process? Kidney Int 64:493–500
Sheng X, Ward MD, Wesson JA (2005) Crystal surface adhesion explains the pathological activity of calcium oxalate hydrates in kidney stone formation. J Am Soc Nephrol 16:1904–1908
Simons SJR, Pratola F, Jones AG, Brunsteiner M, Price SL (2004) Towards a fundamental understanding of the mechanics of crystal agglomeration: a microscopic and molecular approach. Part Part Syst Charact 21:276–283
Stephen H, Stephen T (1963) Solubility of inorganic and organic compounds, vol 1, part 1. Pergamon Press, Oxford, p 251
Vendel M, Rasmuson ÅC (1997) Mechanisms of initiation of incrustation. AIChE J 43:1300–1308
Walker RA, Bott TR (1976) An approach to the prediction of fouling in heat exchanger tubes from existing data. Trans Inst Chem Eng 51:165–167
Werness PG, Brown CM, Smith LH, Findlayson B (1985) EQUIL 2: a basic computer programme for the calculation of urinary saturation. J Urol 134:1242–1244
Zauner R, Jones AG (2000) Determination of nucleation, growth, agglomeration and disruption kinetics from experimental precipitation data: the calcium oxalate system. Chem Eng Sci 55:4219–4232
Acknowledgements
The valuable comments, particularly on the estimation of the critical supersaturation ratio, of Professor D. Kashchiev of the Bulgarian Academy of Sciences and a Leverhulme Visiting Professor at the University of Leeds are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borissova, A., Goltz, G.E., Kavanagh, J.P. et al. Reverse engineering the kidney: modelling calcium oxalate monohydrate crystallization in the nephron. Med Biol Eng Comput 48, 649–659 (2010). https://doi.org/10.1007/s11517-010-0617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-010-0617-y