Abstract
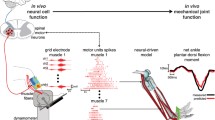
We investigated the parameter identification of a multi-scale physiological model of skeletal muscle, based on Huxley’s formulation. We focused particularly on the knee joint controlled by quadriceps muscles under electrical stimulation (ES) in subjects with a complete spinal cord injury. A noninvasive and in vivo identification protocol was thus applied through surface stimulation in nine subjects and through neural stimulation in one ES-implanted subject. The identification protocol included initial identification steps, which are adaptations of existing identification techniques to estimate most of the parameters of our model. Then we applied an original and safer identification protocol in dynamic conditions, which required resolution of a nonlinear programming (NLP) problem to identify the serial element stiffness of quadriceps. Each identification step and cross validation of the estimated model in dynamic condition were evaluated through a quadratic error criterion. The results highlighted good accuracy, the efficiency of the identification protocol and the ability of the estimated model to predict the subject-specific behavior of the musculoskeletal system. From the comparison of parameter values between subjects, we discussed and explored the inter-subject variability of parameters in order to select parameters that have to be identified in each patient.





Similar content being viewed by others
Notes
This algorithm is based on trust-region method, which uses an approximation of the objective function with a simpler one in the neighborhood around the current variable, called the trust region [31]. For the implementation, Matlab optimization tools were used through the “fmincon” function.
References
Benoussaad M, Hayashibe M, Fattal C, Poignet P, Guiraud D (2009) Identification and validation of FES physiological musculoskeletal model in paraplegic subjects. In: 31st annual international conference of the IEEE engineering in medicine and biology society, pp 6538–6541
Benoussaad M, Poignet P, Guiraud D (2008) Optimal functional electrical stimulation patterns synthesis for knee joint control. In: Intelligent robots and systems IROS. IEEE/RSJ international conference on Nice pp 2386–2391. doi:10.1109/IROS.2008.4651112
Bonnefoy A, Doriot N, Senk M, Dohin B, Pradon D, Chze L (2007) A non-invasive protocol to determine the personalized moment arms of knee and ankle muscles. J Biomech 40:1776–1785
Chae J, Kilgore K, Triolo R, Creasey G, DiMarco A (2004) Functional neuromuscular stimulation. In: DeLisa J, Gans D (eds) Rehabilitation medicine: principles and practice, 4th edn. Philadelphia, J. B. Lippincott Company, pp 1405–1425
Chang YW, Su FC, Wu HW, An K N (1999) Optimum length of muscle contraction. Clin Biomech (Bristol, Avon) 14:537–542
Chia TL, Chow PC, Chizeck HJ (1991) Recursive parameter identification of constrained systems: an application to electrically stimulated muscle. IEEE Trans Biomed Eng 38:429–442. doi:10.1109/10.81562
Chizeck HJ, Chang S, Stein RB, Scheiner A, Ferencz DC (1999) Identification of electrically stimulated quadriceps muscles in paraplegic subjects. IEEE Trans Biomed Eng 46:51–61
Cook C, McDonagh M (1996) Measurement of muscle and tendon stiffness in man. Eur J Appl Physiol Occup Physiol 72:380–382
Crago PE, Peckham PH, Thrope GB (1980) Modulation of muscle force by recruitment during intramuscular stimulation. IEEE Trans Biomed Eng 27:679–684. doi:10.1109/TBME.1980.326592
Delp SL (1990) Surgery simulation: a computer graphics system to analyze and design musculoskeletal reconstructions of the lower limb. PhD thesis, Department of Mechanical Engeneering, Stanford University
Durfee WK, MacLean KE (1989) Methods for estimating isometric recruitment curves of electrically stimulated muscle. IEEE Trans Biomed Eng 36:654–667. doi:10.1109/10.32097
Durfee WK, Palmer KI (1994) Estimation of force-activation, force–length, and force–velocity properties in isolated, electrically stimulated muscle. IEEE Trans Biomed Eng 41:205–216. doi:10.1109/10.284939
Ferrarin M, Iacuone P, Mingrino A, Frigo C, Pedotti A (1996) A dynamic model of electrically activated knee muscles in healthy and paraplegics. chap. Neuroprosthetics from Basic Research to Clinical Applications A. Pedotti, M. Ferrarin, R. Riener, and J. Quintern, Eds. Berlin, Germany: Springer-Verlag pp. 81–90
Ferrarin M, Palazzo F, Riener R, Quintern J (2001) Model-based control of fes-induced single joint movements. IEEE Trans Rehabil Eng 9:245–257
Ferrarin M, Pedotti A (2000) The relationship between electrical stimulus and joint torque: a dynamic model. IEEE Trans Rehabil Eng 8:342–352
Franken DH, Veltink PP, Tijsmans IR, Nijmeijer DH, Boom PH (1993) Identification of passive knee joint and shank dynamics in paraplegics using quadriceps stimulation. IEEE Trans Rehabil Eng 1:154–164
Franken DH, Veltink PP, Tijsmans IR, Nijmeijer G, Boom PH (1995) Identification of quadriceps-shank dynamics using randomized interpulse interval stimulation. IEEE Trans Rehabil Eng 3:182–192
Guiraud D, Stieglitz T, Koch KP, Divoux JL, Rabischong P (2006) An implantable neuroprosthesis for standing and walking in paraplegia: 5-year patient follow-up. J Neural Eng 3:268–275
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet 377:1938–1947
Hatze H (1978) A general myocybernetic control model of skeletal muscle. Biol Cybern 28:143–157
Hatze H (1981) Myocybernetic Control Models of Skeletal Muscle. South Africa: University of South Africa
Hawkins D, Hull M (1990) A method for determining lower extremity muscle-tendon lengths during flexion/extension movements. J Biomech 23:487–494
Hayashibe M, Poignet P, Guiraud D, Makssoud HE (2008) Nonlinear identification of skeletal muscle dynamics with sigma-point kalman filter for model-based fes. In: 2008 IEEE international conference on robotics and automation. Pasadena, CA, USA, pp 2049–2054
Hill A (1938) The heat of shortening and the dynamic constants of muscle. R Soc Lond Proc Ser B 126:136–195
Huxley AF (1957) Muscle structure and theories of contraction. Prog Biophys Biophys Chem 7:255–318
de Leva P (1996) Adjustments to Zatsiorsky–Seluyanov as segment inertia parameters. J Biomech 29:1223–1230
Levy M, Mizrahi J, Susak Z (1990) Recruitment, force and fatigue characteristics of quadriceps muscles of paraplegics isometrically activated by surface functional electrical stimulation. J Biomed Eng 12:150–156. doi:10.1016/0141-5425(90)90136-B
Lin DC, Rymer WZ (1991) A quantitative analysis of pendular motion of the lower leg in spastic human subjects. IEEE Trans Biomed Eng 38:906–918. doi:10.1109/10.83611
Makssoud HE, Guiraud D, Poignet P, Hayashibe M, Wieber PB, Yoshida K, Azevedo-Coste C (2011) Multiscale modeling of skeletal muscle properties and experimental validations in isometric conditions. Biol Cybern 105:121–138. doi:10.1007/s00422-011-0445-7
Morgan DL (1977) Separation of active and passive components of short-range stiffness of muscle. Am J Physiol Cell Physiol 232:C45–C49
Nocedal J, Wright SJ (2006) Numerical optimization, 2nd edn. Springer Series in Operations Research and Financial Engineering, Springer, New York. doi:10.1007/978-0-387-40065-5
Perreault EJ, Heckman CJ, Sandercock TG (2003) Hill muscle model errors during movement are greatest within the physiologically relevant range of motor unit firing rates. J Biomech 36:211–218
Previdi F (2002) Identification of black-box nonlinear models for lower limb movement control using functional electrical stimulation. Control Eng Pract 10:91–99
Riener R, Ferrarin M, Pavan EE, Frigo CA (2000) Patient-driven control of FES-supported standing up and sittingdown: experimental results. IEEE Trans Rehabil Eng 8:523–529
Riener R, Fuhr T (1998) Patient-driven control of FES-supported standing up: a simulation study. IEEE Trans Rehabil Eng 6:113–124. doi:10.1109/86.895956
Riener R, Quintern J (1997) A physiologically based model of muscle activation verified by electrical stimulation. Bioelectrochemistry Bioenerg 43:257–264. doi:10.1016/S0302-4598(96)05191-4
Riener R, Quintern J, Psaier E, Schmidt G (1996) Physiological based multi-input model of muscle activation. Neuroprosthetics: from basic research to clinical applications. Springer, Berlin 12:95–114
Sapio VD, Warren J, Khatib O, Delp S (2005) Simulating the task-level control of human motion: amethodology and framework for implementation. Vis Comput 21:289–302
Schauer T, Negard NO, Previdi F, Hunt K, Fraser M, Ferchland E, Raisch J (2004) Online identification and nonlinear control of the electrically stimulated quadriceps muscle. Control Eng Pract 13:1207–1219
Scheiner A, Stein RB, Ferencz DC, Chizeck HJ (1993) Improved models for the lower leg in paraplegics. In: Engineering in medicine and biology society, the 15th annual international conference of the IEEE, San Diego, CA, USA, pp 1151–1152
Shue G, Crago P E, Chizeck HJ (1995) Muscle-joint models incorporating activation dynamics, moment-angle, and moment-velocity properties. IEEE Trans Biomed Eng 42:212–223. doi:10.1109/10.341834
Stein RB, Zehr EP, Lebiedowska MK, Popović DB, Scheiner A, Chizeck HJ (1996) Estimating mechanical parameters of leg segments in individuals with and without physical disabilities. IEEE Trans Rehabil Eng 4:201–211
Veltink PH, Chizeck HJ, Crago PE, El-Bialy A (1992) Nonlinear joint angle control for artificially stimulated muscle. IEEE Trans Biomed Eng 39:368–380. doi:10.1109/10.126609
Winters J (1990) Hill-based muscle models: a systems engineering perspective, chap 5. In: Winters JM, Woo SY (eds) Multiple muscle systems: biomechanics and movement organization. Springer, New York, pp 69–93
Zahalak GI (1981) A distribution-moment approximation for kinetic theories of muscular contraction. Math Biosci 55:89–114. doi:10.1016/0025-5564(81)90014-6
Zajac FE (1989) Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng 17:359–411
Acknowledgments
The authors would like to thank the patients for their participation and patience, and also Maria Papaiordanidou, Robin Passama and Patrick Benoit for their help during the experiments.
Author information
Authors and Affiliations
Corresponding author
Appendix: Multi-scale muscle model
Appendix: Multi-scale muscle model
The appendix focuses on the contractile element for which a multi-scale approach was used to state, from the microscopic scale using Huxley’s formulation to a macroscopic and controlled muscle model that fully fulfills the well-established muscle law. At the microscopic scale, the authors in [29] started from Huxley’s formulation, which describes the distribution of the fraction of actin–myosin pairs. The key point is the choice of rate functions of attachment f and detachment g of the cross-bridges that allow integration together with a meaningful f and g definitions. Let us begin with the inputs of the model. We designed a model with two separate inputs, i.e. the recruitment rate α and the activation u. The first one is linked to the number of fired motor units deduced from the level of stimulation intensity and pulse width. It is a static relationship described by a sigmoid function [27] and modulated here by the pulse width PW of the electrical stimulus:
where c 1, c 2 and c 3 are parameters which represent the plateau level, maximum slope and inflexion point, respectively. The u function is linked to the calcium dynamics and expresses the rate of the creation or breakage of bridges. It is composed of three phases which are the muscle contraction and relaxation, and the transition between both states. It is defined by the following function [29]:
where \(\Uppi_{\rm c}\) is a function defined as follows:
where t r is the relative time position from the beginning of the transition phase. U c represents the rate of the actin–myosin cycle although U r represents the rate when mainly breakage occurs during the calcium restoring phase in the sarcoplasmic reticulum.
For all subjects, the same values τ c = 30 ms and τ r = 20 ms were obtained through curve fitting of isometric muscle forces under stimulation twitches. The values of U c and U r were fixed for all subject as well to 30 s−1 and 10 s−1, respectively.
From the activation function above and when stimulus is under way, f (although f is always zero) can be defined as follows:
where ξ is the relative position of the myosin head and the closest actin binding site. We consider g to be split in two parts, one linked to the chemical process from the cell at a rate u(t) that depends on the contraction phase and the other one linked to the relative shortening speed that may increase the detachment rate accordingly. It gives (compared to [29] the parameter a = 1):
Our definition is thus linked to the microscopic mechanisms involved within the muscle cell, but another assumption is now needed to change scale and to assess the macroscopic behavior. We do not compute the distribution n of the actin–myosin pairs fraction or express a specific distribution to solve the problem. Instead, we approximate the g function during the contraction phase as follows:
when f ≠ 0 and \(\xi \in [0,1].\) It can be shown that a constant can be added to this formulation without any mathematical change if the definition of g has to be adjusted. This is in fact equivalent to changing the U c and k 0 values (see below). In any case it is consistent with Huxley’s theory, which expresses that g is lower within than outside of this interval. Then we can write the modified g function:
such that f + g no longer depends on the variable ξ. The dynamics of the first two moments of the distribution n is then possible. The detailed computation is available in [29], and the main steps are:
with y linked to both the relative elongation of cross-bridges and relative position of thin and thick filaments, y 0 is the initial position of the spring, k 0 is the scaled stiffness at the sarcomere scale. The dynamics of these moments become:
Due to the wise definition of f and g, f + g does not depend on y, so integration is possible and we obtain:
fl is the force length relationship at the sarcomere scale, which represents the relation between the maximum number of actin–myosin pairs that can bind and the relative length of the contractile element. Then considering that all sarcomeres are identical, going to the fiber scale involves multiplying by a scale factor. And then going to the muscle scale involves scaling of the maximum available force and stiffness. This leads to Eq. (1) with macroscopic parameters which can be obtained by a combination of the microscopic parameters.
Rights and permissions
About this article
Cite this article
Benoussaad, M., Poignet, P., Hayashibe, M. et al. Experimental parameter identification of a multi-scale musculoskeletal model controlled by electrical stimulation: application to patients with spinal cord injury. Med Biol Eng Comput 51, 617–631 (2013). https://doi.org/10.1007/s11517-013-1032-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-013-1032-y