Abstract
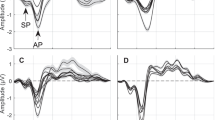
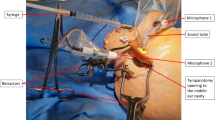
Electrocochleography (ECOG) is a low-amplitude electrophysiological measurement technique primarily used as an assistive tool for the diagnosis of Ménière’s disease. Of the two types of ECOG, transtympanic (TT) and extratympanic (ET), ET-ECOG has gained popularity due to its noninvasive nature; however, it suffers from increased susceptibility to various types of noise, due to the low-signal amplitude (~1 µV scale) associated with the method. Therefore, reliably obtaining ECOG recordings involves an environment that minimally interferes with the recording, a low-noise signal recorder, and a carefully executed recording protocol. We propose a new method that involves a modified ear electrode and electrode placement protocol that offers a solution to reducing noise in ET-ECOG. Noise suppression is achieved by minimizing background biological noise, and thermal noise from electrode impedances, which were identified to be the main contributors to signal degradation in ET-ECOG. Results show that the proposed method yields a >2.6 dB improvement in SNR in comparison with the conventional method (p < 0.05); thus, a SNR obtained with ~880 repetitions using conventional method can be achieved with ~360 repetitions. Improved SNR demonstrate that the proposed method is capable of achieving faster recordings, while maintaining similar or better SNR compared to conventional methods.







Similar content being viewed by others
References
Acharya V (2011) Improving common-mode rejection using the right leg driver amplfier. Application report
Arslan E, Santarelli R, Sparacino G, Sella G (2000) Compound action potential and cochlear microphonic extracted from electrocochleographic responses to condensation or rarefaction clicks. Acta Otolaryngologica 120(2):192–196
Bell SL, Smith DC, Allen R, Lutman ME (2004) Recording the middle latency response of the auditory evoked potential as a measure of depth of anaesthesia. A technical note. Br J Anaesth 92(3):442–445. doi:10.1093/bja/aeh074
Bonucci AS, Hyppolito MA (2009) Comparison of the use of tympanic and extratympanic electrodes for electrocochleography. Laryngoscope 119(3):563–566. doi:10.1002/lary.20105
Brown D (2006) Origins and use of the stochastic and sound-evoked extracellular electrical activity of the auditory nerve. PhD, University of Western Australia
Carpi F, Migliorini S (2009) Non-invasive wet electrocochleography. IEEE Trans Biomed Eng 56(11 Pt 2):2744–2747. doi:10.1109/TBME.2009.2026178
Criswell E (2010) Cram’s introduction to surface electromyography. Jones and Bartlett Learning
Dobbs AK, Krueger WWO, Bishop S (2013) Comparison of transtympanic and extratympanic electrocochleography. IJOHNS Int J Otolaryngol Head Neck Surg 02(05):160–164
Ferraro J (2000) Electrocochleography. Audiology: diagnosis. Thieme, New York
Ferraro JA (2010) Electrocochleography: a review of recording approaches, clinical applications, and new findings in adults and children. J Am Acad Audiol 21(3):145–152. doi:10.3766/jaaa.21.3.2
Ferraro JA, Durrant JD (2006) Electrocochleography in the evaluation of patients with Meniere’s disease/endolymphatic hydrops. J Am Acad Audiol 17(1):45–68
Ferraro JA, Ferguson R (1989) Tympanic ECochG and conventional ABR: a combined approach for the identification of wave I and the I-V interwave interval. Ear Hear 10(3):161–166
Ferraro JA, Thedinger BS, Mediavilla SJ, Blackwell WL (1994) Human summating potential to tone bursts: observations on tympanic membrane versus promontory recordings in the same patients. J Am Acad Audiol 5(1):24–29
Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110(3):577–586. doi:10.1152/jn.00164.2013
Ghosh S, Gupta AK, Mann SS (2002) Can electrocochleography in Meniere’s disease be noninvasive? J Otolaryngol 31(6):371–375
Gibson WP, Beagley HA (1976) Electrocochleography in the diagnosis of acoustic neuroma. J Laryngol Otol 90(2):127–139
Gondran C, Siebert E, Yacoub S, Novakov E (1996) Noise of surface bio-potential electrodes based on NASICON ceramic and Ag-AgCl. Med Biol Eng Comput 34(6):460–466
Hewlett-Packard (1971) Patient safety. Application note AN 718
Hornibrook J, Kalin C, Lin E, O’Beirne GA, Gourley J (2012) Transtympanic electrocochleography for the diagnosis of Meniere’s Disease. Int J Otolaryngol 2012:852714. doi:10.1155/2012/852714
Huigen E, Peper A, Grimbergen CA (2002) Investigation into the origin of the noise of surface electrodes. Med Biol Eng Comput 40(3):332–338
Hwang JH, Ho HC, Hsu CJ, Yang WS, Liu TC (2008) Diagnostic value of combining bilateral electrocochleography results for unilateral Meniere’s disease. Audiol Neurootol 13(6):365–369. doi:10.1159/000136155
Johnson JB (1928) Thermal agitation of electricity in conductors. Phys Rev 32(1):97–109
Krieg SM, Kempf L, Droese D, Rosahl SK, Meyer B, Lehmberg J (2014) Superiority of tympanic ball electrodes over mastoid needle electrodes for intraoperative monitoring of hearing function. J Neurosurg 120(5):1042–1047. doi:10.3171/2014.1.JNS13396
Kumaragamage C, Edwards R, Moussavi Z, Lithgow B Implementation of a gelatin model to simulate biological activity in the inner ear for electrovestibulography (EVestG) validation. In: Engineering in medicine and biology society (embc), 2013 35th annual international conference of the IEEE, 2013. pp 4545–4548. doi:10.1109/EMBC.2013.6610558
Kumaragamage C, Lithgow B, Moussavi Z (2014) Development of an ultra low noise, miniature signal conditioning device for vestibular evoked response recordings. Biomed Eng 13(1):6
McAdams E, Jossinet J (1990) The importance of the electrode skin impedance in high resolution electrocardiography. Automedica 13:187–208
Metting van Rijn AC, Peper A, Grimbergen CA (1990) High-quality recording of bioelectric events. Part 1. Interference reduction, theory and practice. Med Biol Eng Comput 28(5):389–397
Nagel J (2000) Biopotential amplifiers. The biomedical engineering handbook, 2nd edn
Noguchi Y, Nishida H, Komatsuzaki A (1999) A comparison of extratympanic versus transtympanic recordings in electrocochleography. Audiology 38(3):135–140
Purves D (2004) Neuroscience, 3rd edn. Sinauer Associates
Ruth RA, Lambert PR (1989) Comparison of tympanic membrane to promontory electrode recordings of electrocochleographic responses in patients with Meniere’s disease. Otolaryngol Head Neck Surg 100(6):546–552
Schoonhoven R, Prijs VF, Grote JJ (1996) Response thresholds in electrocochleography and their relation to the pure tone audiogram. Ear Hear 17(3):266–275
Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33(34):13686–13694. doi:10.1523/JNEUROSCI.1783-13.2013
Simon MV (2011) Neurophysiologic intraoperative monitoring of the vestibulocochlear nerve. J Clin Neurophysiol 28(6):566–581. doi:10.1097/WNP.0b013e31823da494
Wang LE, Wang Z, Zhang DX, Cao KL (2009) Application of intraoperative round window electrocochleography for screening the patients with auditory neuropathy. Chin Med J 122(8):941–944
Wazen JJ, Emerson R, Foyt D (1997) Intra-operative electrocochleography in stapedectomy and ossicular reconstruction. Am J Otol 18(6):707–713
Webster J (2010) Medical instrumentation application and design 4th ed. Wiley
Winter BB, Webster JG (1983) Driven-right-leg circuit design. IEEE Trans Biomed Eng 30(1):62–66. doi:10.1109/TBME.1983.325168
Yamamoto T, Yamamoto Y (1976) Electrical properties of the epidermal stratum corneum. Med Biol Eng 14(2):151–158
Acknowledgments
The authors acknowledge financial support provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada, Neural Diagnostics Pty Ltd, and MITACS Accelerate Internship program. The work was conducted at both Riverview Health Centre, Winnipeg Manitoba, and at the Department of Electrical and Computer Engineering, University of Manitoba. The authors thank Dr. Roger Edwards for his continued support throughout this work. The authors also thank technical staff members, Allan McKay and Zoran Trajkoski, at the University of Manitoba for their technical support.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumaragamage, C., Lithgow, B. & Moussavi, Z. A new low-noise signal acquisition protocol and electrode placement for electrocochleography (ECOG) recordings. Med Biol Eng Comput 53, 499–509 (2015). https://doi.org/10.1007/s11517-015-1251-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-015-1251-5