Abstract
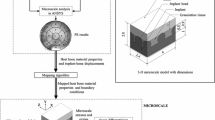
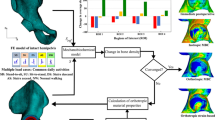
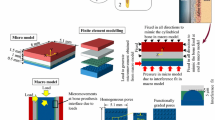
Several mechanobiology algorithms have been employed to simulate bone ingrowth around porous coated implants. However, there is a scarcity of quantitative comparison between the efficacies of commonly used mechanoregulatory algorithms. The objectives of this study are: (1) to predict peri-acetabular bone ingrowth using cell-phenotype specific algorithm and to compare these predictions with those obtained using phenomenological algorithm and (2) to investigate the influences of cellular parameters on bone ingrowth. The variation in host bone material property and interfacial micromotion of the implanted pelvis were mapped onto the microscale model of implant–bone interface. An overall variation of 17–88 % in peri-acetabular bone ingrowth was observed. Despite differences in predicted tissue differentiation patterns during the initial period, both the algorithms predicted similar spatial distribution of neo-tissue layer, after attainment of equilibrium. Results indicated that phenomenological algorithm, being computationally faster than the cell-phenotype specific algorithm, might be used to predict peri-prosthetic bone ingrowth. The cell-phenotype specific algorithm, however, was found to be useful in numerically investigating the influence of alterations in cellular activities on bone ingrowth, owing to biologically related factors. Amongst the host of cellular activities, matrix production rate of bone tissue was found to have predominant influence on peri-acetabular bone ingrowth.








Similar content being viewed by others
References
Anderson AE, Peters CL, Tuttle BD, Weiss JA (2005) Subject-specific finite element model of the pelvis: development, validation, sensitive studies. J Biomech Eng 127(3):364–373
Andreykiv A, van Keulen F, Prendergast PJ (2008) Computational mechanobiology to study the effect of surface geometry on peri-implant tissue differentiation. J Biomech Eng 130(5):051015
Andreykiv A, van Keulen F, Prendergast PJ (2008) Simulation of fracture healing incorporating mechanoregulation of tissue differentiation and dispersal/proliferation of cells. Biomech Model Mechanobiol 7(6):443–461
Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, Duda GN (2001) Hip contact forces and gait patterns from routine activities. J Biomech 34(7):859–871
Carter DR, Blenman PR, Beaupre GS (1988) Correlations between mechanical stress history and tissue differentiation in initial fracture healing. J Orthop Res 6(5):736–748
Checa S, Prendergast PJ (2009) A mechanobiological model for tissue differentiation that includes angiogenesis: a lattice-based modeling approach. Ann Biomed Eng 37(1):129–145
Chou HY, Müftü S (2013) Simulation of peri-implant bone healing due to immediate loading in dental implant treatments. J Biomech 46(14):871–878
Claes LE, Heigele CA (1999) Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech 32(3):255–266
Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P (1998) Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res 355(Suppl):S132–S147
Claes L, Augat P, Suger G, Wilke HJ (1997) Influence of size and stability of the osteotomy gap on the success of fracture healing. J Orthop Res 15(4):577–584
Clarke SG, Phillips ATM, Bull AMJ (2013) Evaluating a suitable level of model complexity for finite element analysis of the intact acetabulum. Comput Methods Biomech Biomed Eng 16(7):717–724
Dalstra M, Huiskes R (1995) Load transfer across the pelvis bone. J Biomech 28(6):715–724
Dar FH, Meakin JR, Aspden RM (2002) Statistical methods in finite element analysis. J Biomech 35(9):1155–1161
Davies JE (1996) In vitro modeling of the bone/implant interface. Anat Rec 245(2):426–445
Davies JE (2003) Understanding peri-implant endosseous healing. J Dent Educ 67(8):932–949
Dickinson A, Taylor A, Browne M (2012) Implant–bone interface healing and adaptation in resurfacing hip replacement. Comput Methods Biomech Biomed Eng 15(9):935–947
Dostal WF, Andrews JG (1981) A three-dimensional biomechanical model of hip musculature. J Biomech 14(11):803–812
Engh CA, Zettl-Schaffer KF, Kukita Y, Sweet D, Jasty M, Bragdon C (1993) Histological and radiographic assessment of well functioning porous-coated acetabular components. A human postmortem retrieval study. J Bone Joint Surg Am 75A(6):814–824
Enns-Bray WS, Owoc JS, Nishiyama KK, Boyd SK (2014) Mapping anisotropy of the proximal femur for enhanced image based finite element analysis. J Biomech 47(13):3272–3278
Funkenbusch PD (2005) Practical guide to designed experiments: a unified modular approach. Marcel Dekker, New York
García-Aznar JM, Kuiper JH, Gómez-Benito MJ, Doblaré M, Richardson JB (2007) Computational simulation of fracture healing: influence of interfragmentary movement on the callus growth. J Biomech 40(7):1467–1476
Ghosh R, Gupta S (2014) Bone remodelling around cementless composite acetabular components: the effects of implant geometry and implant–bone interfacial conditions. J Mech Behav Biomed Mater 32:257–269
Ghosh R, Gupta S, Dickinson A, Browne M (2012) Experimental validation of finite element models of intact and implanted composite hemi-pelvises using digital image correlation. J Biomech Eng 134(2):1–9
Ghosh R, Gupta S, Dickinson A, Browne M (2013) Experimental validation of numerically predicted strain and micromotion in intact and implanted composite hemi-pelvises. Proc Inst Mech Eng H 227(2):162–174
Ghosh R, Mukherjee K, Gupta S (2013) Bone remodelling around uncemented metallic and ceramic acetabular components. Proc Inst Mech Eng H 227(5):490–502
Ghosh R, Pal B, Ghosh D, Gupta S (2015) Finite element analysis of a hemi-pelvis: the effect of inclusion of cartilage layer on acetabular stresses and strain. Comput Methods Biomech Biomed Eng 18(7):697–710
Gómez-Benito MJ, García-Aznar JM, Kuiper JH, Doblaré M (2005) Influence of fracture gap size on the pattern of long bone healing: a computational study. J Theor Biol 235(1):105–119
Hanzlik JA, Day JS (2013) Bone ingrowth in well-fixed retrieved porous tantalum implants. J Arthroplasty 28(6):922–927
Hori RY, Lewis JL (1982) Mechanical properties of the fibrous tissue found at the bone-cement interface following total joint replacement. J Biomed Mater Res 16(6):911–927
Huiskes R, van Driel WD, Prendergast PJ, Søballe K (1997) A biomechanical regulatory model for periprosthetic fibrous tissue differentiation. J Mater Sci Mater Med 8(12):785–788
Isaksson H, van Donkelaar CC, Ito K (2009) Sensitivity of tissue differentiation and bone healing predictions to tissue properties. J Biomech 42(5):555–564
Isaksson H, van Donkelaar CC, Huiskes R, Ito K (2008) A mechano-regulatory bone-healing model incorporating cell-phenotype specific activity. J Theor Biol 252(2):230–246
Isaksson H, van Donkelaar CC, Huiskes R, Yao J, Ito K (2008) Determining the most important cellular characteristics for fracture healing using design of experiments methods. J Theor Biol 255(1):26–39
Isaksson H, Wilson W, van Donkelaar CC, Huiskes R, Ito K (2006) Comparison of biophysical stimuli for mechanoregulation of tissue differentiation during fracture healing. J Biomech 39(8):1507–1516
Jurvelin JS, Buschmann MD, Hunziker EB (1997) Optical and mechanical determination of Poisson’s ratio of adult bovine humeral articular cartilage. J Biomech 30(3):235–241
Khayyeri H, Checa S, Tägil M, Aspenberg P, Prendergast PJ (2011) Variability observed in mechano-regulated in vivo tissue differentiation can be explained by variation in cell mechano-sensitivity. J Biomech 44(6):1051–1058
Kienapfel H, Sprey C, Wilke A, Griss P (1999) Implant fixation by bone ingrowth. J Arthroplasty 14(3):355–368
Lacroix D, Prendergast PJ (2002) Three-dimensional simulation of fracture repair in the human tibia. Comput Methods Biomech Biomed Eng 5(5):369–376
Lacroix D, Prendergast PJ (2002) A mechano-regulation model for tissue differentiation during fracture healing: analysis of gap size and loading. J Biomech 35(9):1163–1171
Lacroix D, Prendergast PJ, Li G, Marsh D (2002) Biomechanical model to simulate tissue differentiation and bone regeneration: application to fracture healing. Med Biol Eng Comput 40(1):14–21
Liu F, Jin Z, Roberts P, Grigoris P (2006) Importance of head diameter, clearance and cup wall thickness in elastohydrodynamic lubrication analysis of metal-on-metal hip resurfacing prostheses. Proc Inst Mech Eng H 220(6):695–704
Liu X, Niebur GL (2008) Bone ingrowth into a porous coated implant predicted by a mechano-regulatory tissue differentiation algorithm. Biomech Model Mechanobiol 7(4):335–344
Montgomery D (2008) Design and analysis of experiments. Wiley, Danver
Morrison ML (2006) Birmingham hip resurfacing system. Adv Mater Processes 164(10):52–53
Mukherjee K, Gupta S (2014) Simulation of tissue differentiation around acetabular cups: the effects of implant-bone relative displacement and polar gap. Adv Biomech Appl 1(2):95–109
Mukherjee K, Gupta S (2015) Bone ingrowth around porous coated acetabular implant: a three-dimensional finite element study using mechanoregulatory algorithm. Biomech Model Mechanobiol 15(2):389–403
Pérez MA, Prendergast PJ (2007) Random-walk models of cell dispersal included in mechanobiological simulations of tissue differentiation. J Biomech 40(10):2244–2253
Phadke MS (1989) Quality engineering using robust design. Prentice-Hall, Engelwood Cliffs
Plackett RL, Burman JP (1946) The design of optimum multifactorial experiments. Biometrika 33(4):305–325
Prendergast PJ, Huiskes R, Søballe K (1997) Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech 30(6):539–548
Puthumanapully PK, Browne M (2011) Tissue differentiation around a short stemmed metaphyseal loading implant employing a modified mechanoregulatory algorithm: a finite element study. J Orthop Res 29(5):787–794
San Antonio T, Ciaccia M, Müller-Karger C, Casanova E (2012) Orientation of orthotropic material properties in a femur FE model: a method based on the principal stresses directions. Med Eng Phys 34(7):914–919
Taddei F, Pancanti A, Viceconti M (2004) An improved method for the automatic mapping of computed tomography numbers onto finite element models. Med Eng Phys 26(1):61–69
Thompson MS, Northmore-Ball MD, Tanner KE (2002) Effect of acetabular resurfacing component material and fixation on the strain distribution in the pelvis. Proc Inst Mech Eng H 216(4):237–245
US Food and Drug Administration (FDA) (2006) Birmingham Hip Resurfacing (BHR) System: Summary of Safety and Effectiveness data http://www.accessdata.fda.gov/cdrh_docs/pdf4/P040033b.pdf. Accessed 21st August 2014
Verhulp E, van Rietbergen B, Huiskes R (2006) Comparison of micro-level and continuum-level voxel models of the proximal femur. J Biomech 39(16):2951–2957
Yew A, Jin ZM, Donn A, Morlock MM, Isaac G (2002) Deformation of press-fitted metallic resurfacing cups part 2: finite element simulation. Proc Inst Mech Eng H 220(2):311–319
Zhang QH, Wang JY, Lupton C, Adegbile PH, Guo ZX, Liu Q, Tong J (2010) A subject-specific pelvic bone model and its application to cemented acetabular replacements. J Biomech 43(14):2722–2727
Acknowledgments
The authors are thankful to Dr. D. K. Nanda and Mr. A. Chattopadhyay of Computer and Informatics Centre, IIT, Kharagpur, for extending computational infrastructure required for the study. The suggestions of Prof. Abhijit Guha and Dr. Souptick Chanda, Department of Mechanical Engineering, IIT, Kharagpur, are greatly appreciated. The authors would also like to thank University of Southampton, UK, for providing CT scan of the patient under the UKIERI British Council Collaborative Research Project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mukherjee, K., Gupta, S. Mechanobiological simulations of peri-acetabular bone ingrowth: a comparative analysis of cell-phenotype specific and phenomenological algorithms. Med Biol Eng Comput 55, 449–465 (2017). https://doi.org/10.1007/s11517-016-1528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-016-1528-3