Abstract
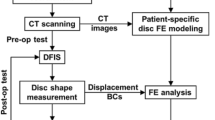
Animal models have been extensively used for the study of degenerative diseases and evaluation of new therapies to stop or even reverse the disease progression. The aim of this study is to reproduce lumbar intervertebral disc degeneration in a rabbit model by performing a percutaneous annular puncture at L4L5 level. The effect of this damage on the spine behaviour was analysed combining three different techniques: imaging processing, mechanical testing and computational modelling. Twenty New Zealand white rabbits were divided into control and experimental groups and followed up during 6 months. Intervertebral disc height, as well as nucleus area and signal intensity, decreased with degeneration while storage and loss moduli increased. Both changes may be related to the loss of water and tissue fibrosis. Similar but slighter changes were reported for adjacent discs. A finite element model was built based on MRI and mechanical testing findings to add new biomechanical information that cannot be obtained experimentally. Four stages were computationally simulated representing the different experimental phases. The numerical simulations showed that compressive stresses in the damaged and adjacent discs were modified with the progression of degeneration. Although extrapolation to humans should be carefully made, the use of numerical animal models combined with an experimental one could give a new insight of the overall mechanical behaviour of the spine.







Similar content being viewed by others
References
Hoy D, March L, Brooks P, Woolf A, Blyth F, Vos T et al (2010) Measuring the global burden of low back pain. Best Pract Res Clin Rheumatol 24:155–165. https://doi.org/10.1016/j.berh.2009.11.002
Vo N, Niedernhofer LJ, Nasto LA, Jacobs L, Robbins PD, Kang J et al (2013) An overview of underlying causes and animal models for the study of age-related degenerative disorders of the spine and synovial joints. J Orthop Res 31:831–837. https://doi.org/10.1002/jor.22204
Urban JPG, Roberts S (2003) Degeneration of the intervertebral disc. Arthritis Res Ther 5:120–130. https://doi.org/10.1186/ar629.
Kääpä E, Holm S, Han X, Takala T, Kovanen V, Vanharanta H (1994) Collagens in the injured porcine intervertebral disc. J Orthop Res 12:93–102. https://doi.org/10.1002/jor.1100120112
Osti OL, Vernon-Roberts B, Fraser RD (1990) 1990 Volvo Award in experimental studies. Anulus tears and intervertebral disc degeneration. An experimental study using an animal model. Spine (Phila Pa 1976) 15:762–767
Olsewski JM, Schendel MJ, Wallace LJ, Ogilvie JW, Gundry CR (1996) Magnetic resonance imaging and biological changes in injured intervertebral discs under normal and increased mechanical demands. Spine (Phila Pa 1976) 21:1945–1951
Reitmaier S, Schuelke J, Schmidt H, Volkheimer D, Ignatius A, Wilke HJ (2017) Spinal fusion without instrumentation—experimental animal study. Clin Biomech 46:6–14. https://doi.org/10.1016/j.clinbiomech.2017.04.008.
Alini M, Eisenstein SM, Ito K, Little C, Kettler AA, Masuda K et al (2008) Are animal models useful for studying human disc disorders/degeneration? Eur Spine J 17:2–19. https://doi.org/10.1007/s00586-007-0414-y
Lauerman W, Platenberg R, Cain J, Deeney V (1992) Age-related disk degeneration: preliminary report of a naturally occurring baboon model. J Spinal Disord 5:170–174
Anderson GD, Li X, Tannoury T, Beck G, Balian GA (2003) Fibronectin fragment stimulates intervertebral disc degeneration in vivo. Spine (Phila Pa 1976) 28:2338–2345. https://doi.org/10.1097/01.BRS.0000096943.27853.BC.
Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A et al (2002) New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine (Phila Pa 1976) 27:2684–2690. https://doi.org/10.1097/00007632-200212010-00007.
Lipson SJ, Muir H (1980) Vertebral osteophyte formation in experimental disc degeneration. Morphologic and proteoglycan changes over time. Arthritis Rheum 23:319–324
Singh K, Masuda K, An HS (2005) Animal models for human disc degeneration. Spine J 5:267S–279S. https://doi.org/10.1016/jspinee200502.016
Sobajima S, Kompel JF, Kim JS, Wallach CJ, Robertson DD, Vogt MT et al (2005) A slowly progressive and reproducible animal model of intervertebral disc degeneration characterized by MRI, X-ray, and histology. Spine (Phila Pa 1976) 30:15–24
Elliott DM, Yerramalli CS, Beckstein JC, Boxberger JI, Johannessen W, Vresilovic EJ (2008) The effect of relative needle diameter in puncture and sham injection animal models of degeneration. Spine (Phila Pa 1976) 33:588–596. https://doi.org/10.1097/BRS.0b013e318166e0a2.
Kim KS, Yoon ST, Li J, Park JS, Hutton WC (2005) Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine (Phila Pa 1976) 30:33–37
Zhou RP, Zhang ZM, Wang L, Huang MJ, Zheng XC, Cui YN et al (2013) Establishing a disc degeneration model using computed tomography-guided percutaneous puncture technique in the rabbit. J Surg Res 181:e65–e74. https://doi.org/10.1016/j.jss.2012.07.027
Kim YE, Goel VK, Weinstein JN, Lim TH (1991) Effect of disc degeneration at one level on the adjacent level in axial mode. Spine (Phila Pa 1976) 16:331–335
Miyamoto K, Masuda K, Kim JG, Inoue N, Akeda K, Andersson GBJ et al (2006) Intradiscal injections of osteogenic protein-1 restore the viscoelastic properties of degenerated intervertebral discs. Spine J 6:692–703. https://doi.org/10.1016/j.spinee.2006.04.014
Leckie SK, Bechara BP, Hartman RA, Sowa GA, Woods BI, Coelho JP et al (2012) Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J 12:7–20. https://doi.org/10.1016/j.spinee.2011.09.011
Gullbrand SE, Ashinsky BG, Martin JT, Pickup S, Smith LJ, Mauck RL et al (2016) Correlations between quantitative T2 and T1p MRI, mechanical properties and biochemical composition in a rabbit lumbar intervertebral disc degeneration model. J Orthop Res 34(8):1382. https://doi.org/10.1002/jor.23269
Hartman RA, Bell KM, Quan B, Nuzhao Y, Sowa GA, Kang JD (2015) Needle puncture in rabbit functional spinal units alters rotational biomechanics. J Spinal Disord Tech 28:E146–E153. https://doi.org/10.1161/CIRCRESAHA.116.303790.The.
Beckstein JC, Sen S, Schaer TP, Vresilovic EJ, Elliott DM (2008) Comparison of animal discs used in disc research to human lumbar disc: torsion mechanics and collagen content. Spine (Phila Pa 1976) 33:E166–E173. https://doi.org/10.1097/BRS.0b013e31824d911c.Comparison.
Schmidt H, Reitmaier S (2012) Is the ovine intervertebral disc a small human one? A finite element model study. J Mech Behav Biomed Mater 17:229–241. https://doi.org/10.1016/j.jmbbm.2012.09.010.
DeVries Watson NA, Gandhi AA, Fredericks DC, Smucker JD, Grosland NM (2014) Sheep cervical spine biomechanics: a finite element study. Iowa Orthop J 34:137–143
Hachem B, Aubin C-E, Parent S (2017) Porcine spine finite element model: a complementary tool to experimental scoliosis fusionless instrumentation. Eur Spine J 26:1610–1617. https://doi.org/10.1007/s00586-016-4940-3
Lotz J, Colliou O, Chin J, Duncan N, Liebenberg E (1998) Volvo Award winner in biomechanical studies. Compresssion-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine (Phila Pa 1976) 23:2493–2506
Marusteri M, Bacarea V (2010) Comparing groups for statistical differences: how to choose the right statistical test? Biochem Med 20:15–32. 10.11613/BM.2010.004
Meyers M, Chawla K (1998) Mechanical behavior of materials. Cambridge University Press, Cambridge
Kim DW, Chun HJ, Lee SK (2015) Percutaneous needle puncture technique to create a rabbit model with traumatic degenerative disk disease. World Neurosurg 84:438–445. https://doi.org/10.1016/j.wneu.2015.03.066
Adams MA, McNally DS, Dolan P (1996) “Stress” distributions inside intervertebral discs. The effects of age and degeneration. J Bone Joint Surg 78:965–972
Iatridis JC, Weidenbaum M, Setton LA, Mow VC (1996) Is the nucleus pulposus a solid or a fluid? Mechanical behaviors of the nucleus pulposus of the human intervertebral disc. Spine (Phila Pa 1976) 21:1174–1184. https://doi.org/10.1097/00007632-199605150-00009.
Adams MA, Roughley PJ (2006) What is intervertebral disc degeneration, and what causes it? Spine (Phila Pa 1976) 31:2151–2161. https://doi.org/10.1097/01.brs.0000231761.73859.2c.
Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, Bishop PB (1990) Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine (Phila Pa 1976) 15:411–415. https://doi.org/10.1097/00007632-199005000-00012.
Inoue N, Espinoza Orias A (2011) Biomechanics of intervertebral disc degeneration. Orthop Clin N Am 42:487–499. https://doi.org/10.1016/j.ocl.2011.07.001.Biomechanics.
Zhou Z, Gao M, Wei F, Liang J, Deng W, Dai X et al (2014) Shock absorbing function study on denucleated intervertebral disc with or without hydrogel injection through static and dynamic biomechanical tests in vitro. Biomed Res Int 2014. https://doi.org/10.1155/2014/461724
Iatridis JC, Setton LA, Weidenbaum M, Mow VC (1997) Alterations in the mechanical behavior of the human lumbar nucleus pulposus with degeneration and aging. J Orthop Res 15:318–322. https://doi.org/10.1002/jor.1100150224
Freeman AL, Buttermann GR, Beaubien BP, Rochefort WE (2013) Compressive properties of fibrous repair tissue compared to nucleus and annulus. J Biomech 46:1714–1721. https://doi.org/10.1016/j.jbiomech.2013.03.034
Detiger SEL, Hoogendoorn RJW, Van Der Veen AJ, Van Royen BJ, Helder MN, Koenderink GH et al (2013) Biomechanical and rheological characterization of mild intervertebral disc degeneration in a large animal model. J Orthop Res 31:703–709. https://doi.org/10.1002/jor.22296
Bron JL, Koenderink GH, Everts V, Smit TH (2009) Rheological characterization of the nucleus pulposus and dense collagen scaffolds intended for functional replacement. J Orthop Res 27:620–626. https://doi.org/10.1002/jor.20789
Costi J, Stokes I, Gardner-Morse M, Iatridis JC (2008) Frequency-dependent behavior of the intervertebral disc in response to each of six degree of freedom dynamic loading: solid phase and fluid phase contributions. Spine (Phila Pa 1976) 33:173–1738. https://doi.org/10.3816/CLM.2009.n.003.Novel.
Leahy JC, Hukins DWL (2001) Viscoelastic properties of the nucleus pulposus of the intervertebral disk in compression. J Mater Sci Mater Med 12:689–692. https://doi.org/10.1023/A:1011212425029.
Galbusera F, Schmidt H, Neidlinger-Wilke C, Wilke H-J (2011) The effect of degenerative morphological changes of the intervertebral disc on the lumbar spine biomechanics: a poroelastic finite element investigation. Comput Methods Biomech Biomed Eng 14:729–739. https://doi.org/10.1080/10255842.2010.493522
Galbusera F, Schmidt H, Neidlinger-Wilke C, Gottschalk A, Wilke HJ (2011) The mechanical response of the lumbar spine to different combinations of disc degenerative changes investigated using randomized poroelastic finite element models. Eur Spine J 20:563–571. https://doi.org/10.1007/s00586-010-1586-4
Ruberté LM, Natarajan RN, Andersson GB (2009) Influence of single-level lumbar degenerative disc disease on the behavior of the adjacent segments—a finite element model study. J Biomech 42:341–348. https://doi.org/10.1016/j.jbiomech.2008.11.024
Malandrino A, Pozo JM, Castro-Mateos I, Frangi AF, van Rijsbergen MM, Ito K et al (2015) On the relative relevance of subject-specific geometries and degeneration-specific mechanical properties for the study of cell death in human intervertebral disk models. Front Bioeng Biotechnol 3:5. https://doi.org/10.3389/fbioe.2015.00005
van Rijsbergen MM, Barthelemy VMP, Vrancken ACT, Crijns SPM, Wilke HJ, Wilson W et al (2016) Moderately degenerated lumbar motion segments: are they truly unstable? Biomech Model Mechanobiol:1–11. https://doi.org/10.1007/s10237-016-0835-9
Grauer JN, Erulkar JS, Patel TC, Panjabi MM (2000) Biomechanical evaluation of the New Zealand white rabbit lumbar spine: a physiologic characterization. Eur Spine J 9:250–255
Moramarco V, Pérez del Palomar A, Pappalettere C, Doblaré M (2010) An accurate validation of a computational model of a human lumbosacral segment. J Biomech 43:334–342. https://doi.org/10.1016/j.jbiomech.2009.07.042
Cegoñino J, Moramarco V, Calvo-Echenique A, Pappalettere C, Pérez Del Palomar A (2014) A constitutive model for the annulus of human intervertebral disc (IVD): implications for developing a degeneration model and its influence on lumbar spine functioning. J Appl Math 2014. https://doi.org/10.1155/2014/658719
Funding
This work was supported by the Spanish Ministry of Economy and Competitiveness through projects DPI 2016-79302-R and by the Spanish Ministry of Education, Culture and Sports (Grant FPU13/01070).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest should be declared by any of the authors.
Ethical approval
This study was carried out in strict accordance with the recommendations in the Royal Decree 1201/2005 of 10 October 2005 (BOE from Oct. 21) on protection of animals used for experimentation and other scientific purposes. Experimental protocols were approved by the Committee on the Ethics of Animal Experiments of Minimally Invasive Surgery Centre Jesús Usón and by the Council of Agriculture and Rural Development of the Regional Government of Extremadura, Spain.
Electronic supplementary material
Supplementary material Fig. 1
Surgical technique: The needle was inserted, in a percutaneous manner, at 30–35 mm right to the midline spinous process and with an angle of 35–40° with respect to the horizontal plane. The penetration depth was checked under fluoroscopic control until reaching the centre of the IVD. (GIF 62 kb)
High resolution image
(EPS 17646 kb)
Supplementary material Fig. 2
FE results-Tensile stresses: Comparison between the maximal principal stresses at the four stages ((1) Pre-operatively, (2) post-operatively, (3) 3 months after the surgery and (4) 6 months after the surgery) at the punctured level (L4L5) and its adjacent ones (L3L4 and L5L6). (a) Maximal principal stresses variation across a laterolateral line that lies on a frontal cut of the intervertebral disc. The discontinuities are due to the puncture. Data shown for instant and transient response. (b) Colour maps of maximal principal stress distribution after loading and after relaxation period. (PSD 926 kb)
Rights and permissions
About this article
Cite this article
Calvo-Echenique, A., Cegoñino, J., Correa-Martín, L. et al. Intervertebral disc degeneration: an experimental and numerical study using a rabbit model. Med Biol Eng Comput 56, 865–877 (2018). https://doi.org/10.1007/s11517-017-1738-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11517-017-1738-3